A random selection of new technology companies in California that are positive changemakers. The tech scene in California is booming, and so is California’s increasing population in 2024, while California became the 4th largest economy on the planet in 2025, surpassing Japan. If you believe the American mythology that technologies and start-ups are created by college dropouts, think again. You’ll see, by far, most are created by college grads and many by doctors of philosophy. 62% of Unicorn founders held post-graduate degrees, most are in California, over 40 years old, and UC Berkeley, followed by Stanford, are the leading schools for startup founders. If you’re listening to Peter Thiel (who has undergad and law degrees) telling you not to go to college, a failed idea, look at this list of founders, and think again. Indeed, California has the best universities on the planet, including UC Berkeley, UCSD, Stanford, UCLA, and Caltech, which are supporting the California innovation hub, and leading to upward mobility of many. For more than two decades, the University of California has been the leading university patent owner and among the top 100 global patent holders.
In the first half of 2025, roughly 68% of all U.S. startup funding went to California-headquartered companies. If you using the internet, ChatGPT, drive a Tesla or Lucid EV, use PowerPoint, search the web using your Apple computer, an Intel-based computer, buy products on ebay, using a computer or phone screen (quantum dots), store data using DataBricks, integrated circuits, use lithium-ion batteries, use PCR for genetic analysis, RISC computer chips, use an Intel processor, WYSIWYG software editing or Oracle software, immunotherapy for cancer (checkpoint inhibitors), you’re using technology created, or partially created, at UC Berkeley or by UC Berkeley grads. And if you’re using Google, you’re using a product developed by Stanford and Berkeley grads, and all of these technologies were developed with government funding and support. Using fiberoptics and satellite links? They are dependent on technology developed at Caltech by a Caltech grad. So is VLSI. You’ll see in the figure below that San Francisco and California are still, by far, the world’s leader in tech innovation. As always, San Francisco is the hub, and the city’s tech scene is booming, with people arriving from around the world. Let’s look at the new companies and their technologies now arising in California.
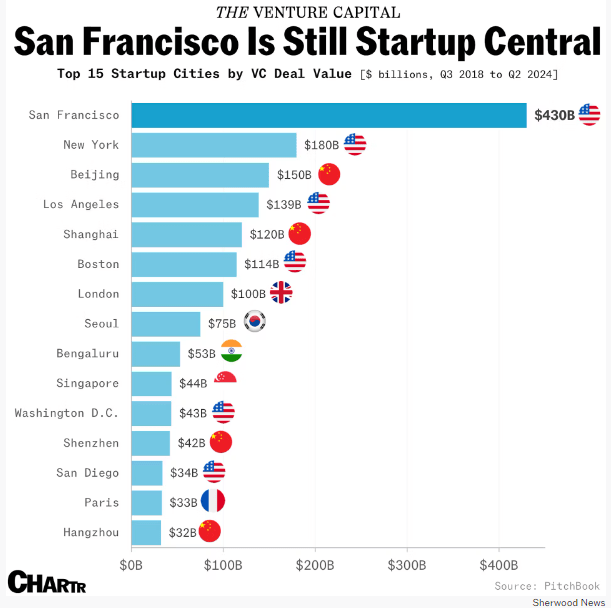
Three of the world’s top 15 startup cities are in California. San Francisco is #1, Los Angeles is #4, and San Diego is #13. No cities in Texas or Florida make the list. New York, Boston, and D.C. also make the list. Note – all are progressive US cities led by Democrats, and with first-rate universities.
Why do tech companies start or move to California? Because doing so raises their chance of success. Even the Imperial College London, one of the UK’s preeminent universities, and the Wharton School of Univ Pennsylvania, have started tech and educational hubs in San Francisco, CA. They join the massive intellectual power of California with its unparalleled universities and R&D centers (both public and private) that drive innovation. New York is similar, but other places, like Texas and Florida, don’t get it. TX and FL try to lure companies with tax breaks and gifts, further eroding their ability to develop educational hubs, that just don’t work. Austin, TX is an example of how not to build a tech hub.
The following list is some of what is happening in California. First, let’s look at some of the key academic research institutions in California that educate the tech leaders and often develop the technologies used by tech companies.
California Academic Research Institutions
UC Berkeley (Berkeley, CA) – This is the most innovative university on the planet. The #2 school, behind Harvard, for producing Nobel Laureates. Faculty Ashok Gadgil, Jennifer Doudna, Chenming Hu have won National Medals of Technology and Innovation, and David Patterson, Richard Karp, William Kahan, Mauel Blum, Silvio Micali, Shafi Goldwasser, and Micael Stonebraker have won the Turing Award. Whether it’s atomic power, nMRI, the 3-D transistor, the LASER, neuroplasticity, integrated circuits, autonomous-driving, SPICE to analyze integrated circuits, CRISPR, RISC computing, CETUS – the first biotech company, the Cyclotron, MEMS, radioactive carbon dating, lithium ion chemistry, all were discovered/invented on the UC Berkeley campus. Dr. Ion Stoica, professor in EECS, has cofounded 4 highly succesful, innovative companies: DataBricks, Anyscale, Conviva, and Opaque Systems. Databricks and Anyscale are unicorns.
Since 1959, the UC Berkeley Space Sciences Laboratory (SSL) has led the nation in the exploration of our atmosphere and deep space. The Space Sciences Laboratory (SSL) at UC Berkeley is one of only two university-based institutions in the U.S. capable of executing the full NASA mission lifecycle. SSL has led over a dozen NASA missions, taking them from concept to delivery at the launch pad to mission operation and scientific analysis. SSL provides engineering expertise to design, build, and test instrumentation for a range of space and ground-based projects. They’ve managed world-class science missions and operated spacecraft throughout our solar system. Their mission operations capabilities include a newly refurbished, all-digital mission operations center, and state-of-the-art facilities supporting cleanroom fabrication and assembly, along with environmental testing.
Nobel Laureate, Prof. Dr. Jim Allison, Ph.D., professor at UC Berkeley developed the now widely used checkpoint inhibitors, immunotherapy, for cancer treatment. This was a paradigm shift for treating cancer, neither destroying cells with chemo or irrdatiation that detstroys your own cells, nor jacking-up the immune system so that it destoys the cancer cells along with your own cells, checkpoint inhibitors renormalize your immune system so that it can attack the cancer cells.
Reading this on a computer or phone screen? Then your benefiting from Quantum Dots developed at UC Berkeley by Prof. Dr. Paul Alivisatos, PhD, a Berkeley grad who has won many awards, and his research team. Nanosys in Milpitas, CA is a manufacturer of quantum dots with over 650 patents and was cofounded by Prof. Dr. Alivisatos. Alivisatos’ other quantum dot company—Quantum Dot Corp., which he cofounded in 1998 to commercialize use of quantum dots for luminescent labeling of biological tissues—has since been acquired, and that technology is now found in biological and medical imaging tools made by Thermo Fisher Scientific. UC Berkeley faculty and alumni, and scientists at the Lawrence Berkeley National Laboratory (Berkeley Lab) have discovered more than 20 chemical elements on the periodic table, many of which are critical to new technologies. Berkeley’s grads have invented the computer mouse, print-based batteries, PCR, wetsuits, the lie detector, the modern lithium-ion EV (Tesla). Berkeley Lab is part of the UC Berkeley campus.
Berkeley National Labs (Berkeley, CA) – A national R&D lab managed by UC and affiliated with UC Berkeley, this institute is a wellspring of fundamental research and development leading to new commercial technologies. For example, one of the research teams discovered and published in 2025 that that atoms in semiconductors will arrange themselves in distinctive localized patterns that change the material’s electronic behavior. This discovery provides a foundation for designing specialized semiconductors for quantum-computing and optoelectronic devices.
Stanford (Stanford, CA) – The #6 school in producing Nobel Laureates. The current Nividia chips used to power Chat AI platforms were developed by Jonah Alben who has BSCSE and MSEE degrees from Stanford University. Recombinant DNA technology was developed here.
Caltech (Pasadena, CA) – The #7 school in producing Nobel Laureates. Caltech alum Dr. William Shockley, Ph.D. co-invented the transistor. Prof Dr Carver Mead, PhD received the National Medal of Technology in 2002 for his work in microelectronics. He invented Neuromorphic Engineering.
UC San Diego (San Diego, CA) – The #17 school in Nobel Laureates. Two photon absorption was discovered by Nobel Laureate, Prof. Dr. Maria Goeppert-Mayer, PhD while a professor at UCSD. Also, Nobel Laureate, Prof. Dr. Roger Tsien, Ph.D., was awarded the prize for his work on on GFP. This phenomenon underlies many imaging processes. UCSD is ranked #8 in the world for innovation among universities. The Fusion Engineering Institute is located on campus, in collaboration with San Diego’s General Atomics.
UCLA (Los angeles, CA) – The #20 school in producing Nobel Laureates. The nicotine patch and CT and PET Scans were developed at UCLA. The internet was first implemented at UCLA.
UC Santa Barbara (Santa Barbara, CA) – # 28 for Nobel Laureates. Atomic Force Microscopy was invented here.
UCSF (San Francisco, CA)- #32 for Nobel Laureates.
USC (Los Angeles, CA) – #33 for Nobel Laureates. Domain Name System (DNS) was invented at USC. USC had the first operational quantum computing system in academia in 2011.
UC Santa Cruz (Santa Cruz, CA) – UCSC scientists released the first complete human genome sequence, advancing understanding of genetic diseases, human diversity, and evolution. Then they led the human pangenome project and the complete sequencing of the human Y chromosome, and made the first detection of carbon dioxide in an exoplanet atmosphere using the James Webb Space Telescope.
UC Irvine (Irvine, CA) #44 for Nobel Laureates.
UC Davis (Davis, CA) – Began as an ag station for UC Berkeley and notably saved the French wine industry from Phylloxera, later became UC Davis and known for crop improvements.
UC Riverside (Riverside, CA) – Leading the revolutionary development of electric-agriculture, a sustainable and cost-efficient method to grow crops. Co-developed by Prof. Dr. Robert E. Jinkerson, PhD.
UC Merced (Merced, CA) – Known for environmental sciences, they are spearheading a project to cover the vast array of canals in California with solar panels, yielding cooler solar panel temperatures that are therefore more efficient, while decreasing evaporation from the canals.
Wharton San Francisco (San Francisco, CA) – Wharton School’s California campus.
Imperial Global College San Francisco (San Francisco, CA) – The prestigious UK school’s campus in SF.
Stand.Earth (San Francisco, cA) – Environmental advocacy.
Djerassi Resident Artists Program – (Woodside, CA) – Artists, writers, musicians, and scientists come to the institute to think, learn, and create. Notable alumni include Prof Dr. Peter Walter, PhD of UCSF, awarded many times for his work on how proteins are processed in our bodies.
Tech Startups
Chemistry
Lilac Soultion (Oakland, CA) – Lilac is a direct lithium extraction technology company that enables producers to extract lithium from brine resources. Founder and Chief Technology Officer Dr. Dave Snydacker, Ph.D. has a PhD in materials engineering from Northwestern University, where his research focused on lithium-ion cathode materials and solid-state lithium metal batteries.
Ammobia (San Francisco, CA) – Ammobia’s process to make ammonia runs at around 150°C cooler and at 10x lower pressure than current technologies. As a result, plants that adopt the technology produce less pollution, even if they don’t ditch fossil fuels in their process. Founders Dr. Tristan Gilbert earned his PhD in Mechanical Engineering from Stanford University, and Karen Baert has MBA from Stanford University (2020-2022); Bachelor’s in Mechanical Engineering – Chemical Technology at KU Leuven (2012-2015); Master’s in Renewable Energy, Technical University of Berlin (2015-2017).
Coreshell Technologies (San Leandro, CA) – Founded in January 2017 by UC Berkeley alumni Jonathan Tan and Roger Basu, part of UC Berkeley’s Skydeck incubator, Coreshell Technologies produces a nanolayer thin-film coating that fits into existing manufactured batteries. The technology could make rechargeable batteries last longer.
NextSet Materials (Berkeley, CA) – Developing a chemistry to make plastics recyclable. Founded by Dr. Yasmeen Alfaraj, Ph.D., a grad of UC Berkeley and MIT.
Molton Industries (Oakland, CA) – Molten has developed a process that not only enables the domestic production of graphite, but also at a lower cost, and while creating a highly valuable hydrogen co-product. Founders Dr. Kevin Bush has a PhD in Materials Science from Stanford University, and Dr. Caleb Boyd studied Mechanical Engineering and Materials Science as an undergraduate at UC Berkeley, during which he took time off to work in product design at Apple. Caleb received his PhD from Stanford University.
Perlumi (Berkeley, CA) – Improved photosynthesis will sustainably supply the food, feed, fiber and materials a bountiful future requires. Founded by Dr. Chris Eiben, PhD, who earned his Ph.D. in Bioengineering at UC Berkeley, focusing on synthetic metabolism and protein engineering in Jay Keasling’s lab and dr. Michael Dougherty, PhD, who earned a B.S. in microbiology from Penn State and a Ph.D. in microbiology from UW-Madison. He worked on engineering protein transcription factors during a postdoctoral appointment at CalTech, and then worked on biofuels research at the Joint Bioenergy Institute at UC Berkeley.
Azolla (Oakland, CA) – Using carbon to make textiles. Azolla’s novel approach uses a genetically engineered bacterium that converts CO2 directly into pure biomaterial via photosynthesis, offering significant process efficiencies and potential for rapid adoption without downstream supply chain disruptions. Founders Lubica Hanacek and her husband Milan are grads of Kent State.
Aepnus (Berkeley, CA) – Aepnus electrifies and decarbonizes the production of a variety of commodity chemicals. Their ultra-efficient electrolyzers run on renewable electricity to process critical minerals, reagents, or metals at lower costs and associated emissions than the existing technologies. Founded by Dr. Lukas Hackl who has a PhD from UC Berkeley and Dr. Bilen Akuzum who has a PhD from Drexel Univ. and was a postdoc at UC Berkeley.
Serinus Labs (Berkeley, CA) – Ultra-low power gas sensors for emerging applications in electric mobility, atmospheric monitoring, energy storage and distribution. Using proprietary technology, their sensors can simultaneously sense several gases at trace levels with unmatched precision, stability and reliability. They spun off from the Berkeley Sensor & Actuator Center (BSAC) at UC Berkeley.
Geltor (Berkeley, CA) – Bespoke protein production. Founders Drs. Nick Ouzounov, PhD and Alex Lorestani, PhD met as Ph.D. students in their molecular biology lab at Princeton.
Magrathrea (Oakland, CA) – Developing innovative technology for the production of carbon neutral light metal from seawater, has partnered with a multinational automotive manufacturing company to deploy US-made light metal in current and future vehicle products. Backed by the US-DOD. Founded by two chemical engineers.
Lyten (San Jose, CA) – Lyten is a supermaterial applications company. They pioneer in Three-Dimensional Graphene, a supermaterial that can be infinitely tuned to exhibit a unique combination of disruptive properties. They use 3D Graphene’s properties to build products that address some of industry’s greatest challenges. Greater strength. Lighter weight. Increased conductivity. Reduced carbon footprint. Batteries are one of the products they make. Founded by William Wraith III, a Stanford grad; Dan Cook a Stanford grad; Lars Herlitz, a grad of University of Linköping.
Circularity Fuels (Palo Alto, CA) – Aviation fuel from manure. Has made a compact reactor for converting biogas into synthesis gas, that can be used for the production of aviation fuel. Founded by Dr. Stephen Beaton, PhD – chemstry doctorate from Univ. Oxford.
Atoco (Irvine, CA) – To mitigate climate change, they capture carbon dioxide from industrial emissions and directly from the atmosphere. And to help resolve water scarcity, they capture clean water directly from the atmosphere. The comapnay was founded by and the technology was developed by Prof. Dr. Omar Yaghi, Ph.D., professor of chemistry at UC Berkeley.
EnergySourceMinerals (San Diego, CA) – They’ve developed technology to extract lithium from brines. Already they have licensed-out their technology. Commercialization of their processes is underway at the Salton Sea in Imperial County, California, which is one of the largest lithium deposits in the world. Dr. David Deak, Ph.D. is their technology leader. He was educated at the University of Toronto and Oxford University
Profluent Bio (Berkeley, CA) – Develops machine learning models that can read and write biomolecules for human health and industrial applications. Cofounded by Dr. Ali Madani, PhD from UC Berkeley.
Unigrid Battery (San Diego, CA) – They make sodium-ion batteries, which use abundant resources, are lower-cost, safer, and just as powerful as any other battery. Cofounded by Dr. Darren Tan, PhD, a doctorate in chemical engineering from UCSD and Dr Erik Wu, PhD, a doctorate in chemical engineering from UCSD.
Fluid Efficiency (Pasadena, CA) – Manufacturer of lubricant molecules designed to make fuels and oils safe and easy to move. Cofounded by Dr. Simon Jones, Ph.D. (from Oxford), Dr. Ming-Hsin Wei, Ph.D. (CalTech professor), and Dr. Julie Kornfield, Ph.D (CalTech professor).
Thintronics (Berkeley, CA) – The complex components of a single computer chip contain layers of microscopic components linked to one another through highways of copper wires, some barely wider than a few strands of DNA. Nestled between those wires is an insulating material called a dielectric, ensuring that the wires don’t touch and short out. Zooming in further, there’s one particular dielectric placed between the chip and the structure beneath it; this material, called dielectric film, is produced in sheets as thin as white blood cells. MIT Technology Review has a great article about Thintronics. Founder and CEO Dr. Stefan Pastine Ph.D is an alum of Columbia Univ.
Intropic Materials (Oakland, CA) – Making plastic bottles that recycle themselves under conditions of increased heat and moisture. Founder and CEO Dr. Aaron Hall, Ph.D., founded Intropic Materials while earning his PhD in Materials Science and Engineering at UC Berkeley.
Aepnus Technologies (Oakland, CA) – They’ve developed a low-cost electrolysis system that can produce commodity chemicals across sectors using renewable electricity. They’re casting off the old way of doing things – using fossil-based feedstocks and fossil-based heat for chemical production. Today, they’re working on the conversion of sulfate salts into high concentration sulfuric acid and metal hydroxides for the battery industry. Cofounded by Dr. Lukas Hackl, Ph.D., a grad of UC Berkeley, and Dr. Bilen Akuzum, PhD, a postdoc at UC Berkeley.
Capture 6 (Berkeley, CA) – A public benefit company, technology that removes CO₂ from the atmosphere while producing additional freshwater for local communities. Founder Dr. Ethan Cohen-Cole, Ph.D, attained his doctorate at the Univ Wisonsin.
Captura (Pasadena, CA) – Their process uses renewable energy to remove CO2 from seawater and amplify the ocean’s natural removal of carbon from the atmosphere — all with no additives or by-products. This is a spin-out of Caltech, founded by Dr CX Xiang, Ph.D., (Ph.D. from UC Irvine) a Research Professor of Applied Physics & Materials Science at Caltech, where the proprietary membrane technology used by Captura was developed. He is also the lead researcher at several U.S. Department of Energy (DOE) ARPA-E studies, and by Dr Harry Atwater, Ph.D. (Ph.D. from MIT), a Professor of Applied Physics & Materials Science at Caltech. He is an experienced technology entrepreneur and a long-time pioneer in photovoltaics. Harry is Director of the DOE Energy Innovation Hub and a member of the National Academy of Engineering.
Hyfe (San Francisco, CA) – Converting plant waste to industrial chemicals. Unlike traditional biorefineries, Hyfé leverages food processing waste, such as grape pomace and carrot peels, which are rich in high-value specialty chemicals. cofounders Michelle Ruiz has a BS in chemical engineering from Carniege Mellon and Andrea Shoen is a grad of Northwestern University.
Ebb Carbon (San Carlos, CA) – Their approach boosts the pH of the water, shifting dissolved CO2 into the more stable bicarbonate and carbonate ions. Founded by Ben Tarbell, who has degrees in engineering and business from Stanford and Cornell, and Dave Hegeman who has two BS degrees in Marine Engineering and in Mechanical Engineering from the California Maritime Academy.
Evoloh (Santa Clara, CA) – They build the highest throughput electrolyzer stack factories in the world. Founded by Dr. Jimmy Rojas, PhD, who has a Ph.D. in hydrogen production and energy systems from Stanford University.
Oberon Fuels (San Diego, CA) – Manufacturer of renewable dimethyl ether fuel intended to address the growing problems of emissions and particulate matter in cities. The company’s fuel utilizes locally available organic wastes to produce low-carbon, zero-soot dimethyl ether fuel, an alternative to diesel that has applications in the trucking and heavy equipment industry. Cofounder Elliot Anise-Hicks is a grad of MIT.
Cloud Water Filters (San Diego, CA) – Clean, alkaline water filtering system.
Sierra Energy (Rocklin, CA) – Sierra Energy is focused on the development of gasification, a technology that turns trash into energy without burning. Founder Mike Hart is a grad of UC Davis.
Astral Materials (Mountain View, CA) – “Manufacturing Materials that Cannot be Made on Earth.” Cofounded by Dr. Jessica Frick, Ph.D. a grad of Princeton and Prof. Dr. Debbie Senesky, PhD a prof at Stanford and PhD grad of UC Berkeley.
Spiral Wave (San Fransciso, CA) – Sustainable platform intended to remove atmospheric carbon dioxide at a giga-scale for a clean environment. The company uses plasma technology that not only removes CO2 but also fosters a circular economy and covert it into methanol. Cofounded by two engineers, Abed Bukhari and Adam Awad.
Aizen Therapeutics (San Diego, CA) – They mak mirror proteins, i.e. fully D-amino acid peptides that represent a vast, unexplored chemical space beyond naturally occurring peptides and proteins. The mirror proteins don’t degrade as quickly by the body’s natural proteases because the enzymes in the body don’t know how to conform to these right-handed orientations. They are also not recognized by the immune system so as not to induce an immune response. Cofounded by Prof. Dr. David Van Valen MD, PhD of Caltech, a grad of MIT (BS), Caltech (PhD), and UCLA (MD), and Ajay Kshatriyaan who has a B.S. in chemical engineering from U.C. Berkeley, M.S. in engineering from Stanford University, and an MBA from Berkeley-Haas School of Business.
Origin Materials (Sacramento, CA) – sustainable and performance-enhanced solutions for improving recycling and circularity, including its all-PET caps and closures, as well as low-carbon material solutions for a wide variety of products and applications. The company just announced it’s discovered a novel way to produce plastics using bio-based feedstocks, like sawdust, old cardboard, and wood chips. Founders John Bissell has a B.S. in Chemical Engineering from the University of California, Davis and Ryan Smith received a B.S. in Chemical Engineering from the University of California, Davis.
Universal Fuel Technologies (Los Altos, CA) – Developer of chemical technology designed to make sustainable aviation fuel (SAF), gasoline, LPG (liquefied petroleum gas), (benzene, toluene and xylenes) BTX from alcohols, ethers, renewable naphtha and light olefins. The company’s technology converts various feeds into drop-in fuels or chemicals, enabling clients to manage the speed of their energy transition and be less dependent on a specific feedstock. Cofounded by Dr. Zachary Schmidt who has a Ph.D. in chemical engineering from Princeton.
HOPO Therapeutics (Berkeley, CA) – HOPO Therapeutics developed HOPO-101, a heavy metal chelating agent that, if approved, would be the first orally available treatment for exposure to heavy metals and radioactive actinide elements such as plutonium, americium, curium, and uranium. Founded by Dr Julian Rees, Ph.D.
Aionics (Palo Alto, CA) – Artificial intelligence and physics-based simulation to design new, customized electrolytes for high performance electrochemical systems: electric vehicle and aerospace batteries, long-duration energy storage systems, decarbonized manufacturing. Founder Dr. Austin Sendek, PhD was educated at UC Davis and Stanford, Dr. Lenson Pellouchoud, PhD a grad of Brown and Stanford, and Prof. Dr. Venkat Viswanathan, PhD of the U. Michigan.
Nucleus Biologics (San Diego, CA) – They make recombinant proteins.
Chilldyne (San Diego, CA) – Liquid cooling systems for all applications, including aerospace and computers. Founded by Dr. Steve Harrington, PhD a UCSD grad in aerospace negineering and instructor at UCSD.
Correlia Biosystems (Berkeley, CA) – Correlia Biosystems is a UC Berkeley spinoff company based in Berkeley, CA. Correlia develops innovative microscale tools that accelerate rapid quantification of biomolecules. Founder Dr. Akwasi Apori, PhD, completed his PhD in Bioengineering at UC Berkeley/UCSF where he developed microscale assays for quantitative biology. He previously worked as a systems engineer at Boeing Satellite Systems and was a founder and principal investigator at Quantsupport. He obtained his BS/MS in Aeronautics and Astronautics from MIT.
Frontier Aerospace (Santa Clarita, CA) – Propulsion systems for aerospace. Founded by Jim McKinnon an engineering grad of Cal Poly.
HoneyBee Robotics (Altadena, CA) – Motion systems for aerospace.
Concrete AI (Los Angeles, CA) – Concrete Copilot delivers concrete producers a simple, fast, and flexible decision-making tool to optimize concrete performance, cost-savings, and carbon reductions. Founded by Prof. Dr. Mathieu Bauchy, PhD of UCLA.
Equatic Tech (Santa Monica, CA) – Equatic catalyzes and powers the green economy. Their seawater electrolysis couples carbon dioxide removal (CDR) from the atmosphere with the production of green hydrogen at low cost. Founded Prof. Dr. Gaurav N. Sant, PhD of UCLA.
Carbon Built (Torrance, CA) – Making ultra-low carbon concrete. Founded Prof. Dr. Gaurav N. Sant, PhD of UCLA.
AirMyne (Berkeley, CA) – Liquid solvent capture agent to capture carbon dioxide from the air. The carbon dioxide-rich liquid is then funneled to a desorption unit, where it is heated, releasing a pure stream of carbon dioxide, ready to be locked away. Founder Sudip Mukhopadhyay is a grad of UC Berkeley and Mark Cyffka is a grad of Harvey Mudd College (near Los Angeles).
Vesta (San Francisco, CA) – Vesta adds a carbon-removing sand made of the natural mineral olivine to coastal systems. This nature-based climate strategy reduces ocean acidity and removes carbon dioxide permanently. Founder David Sneider has a BS from Indiana University, Kelly Erhart is a grad of University of Central Florida, and Tom Green has a BA in Biological Sciences from Oxford University and an MBA from Harvard Business School.
EpicCleanTech (San Francisco, CA) – On site water recycling technology. Founded by Aaron Tartakovsky, a grad of TelAviv University and Igor Tartakovsky, who has BS and MS degrees from Osessa University.
Sway (San Leandro, CA) – Plastics made from sustainbly produced seaweed. Founders Julia Marsh is a grad of Brown Univ and Matt Mayes is a grad of UC Berkeley.
Heirloom Carbon (Brisbane, CA) – Limestone is an abundant and inexpensive rock that captures massive amounts of CO2 from the air over years in a process known as carbon mineralization. Heirloom’s technology accelerates this natural process to just days. Founders Shashank Samala is a Cornell grad and Dr. Noah Mcqueen received a B.S. in Chemical Engineering from Colorado School of Mines in May 2018 and a PhD from the University of Pennsylvania.
Clarity Tech (Los Angeles, CA) – Developer of an air capture system designed to reduce carbon dioxide from the atmosphere. The company’s technology captures the increasing amounts of carbon dioxide, enabling individuals to get a better and more sustainable environment. Founder Glen Meyerowitz has a BS from Yale and MS from UCLA.
H2-MOF (Irvine, CA) – safe and efficient hydrogen storage that doesn’t require liquefaction or high pressure using chemicals called metal-organic frameworks (MOFs). Microporous crystalline MOFs are formed by the self-assembly of inorganic metal clusters and organic linkers and can easily bind and release hydrogen. Founded by Prof. Dr. Omar Yagi, PhD of UC Berkeley, and Prof. Dr. Fraser Stoddart, PhD who was associated with a number of academic institutions, including the California Nanosystems Institute at UCLA/UC Santa Barbara.
Chemix (Sunnyvale, CA) – AI platform for battery development. Founded by Dr. Kaixiang Lin, PhD, grad of Harvard and Dr. Jason Koeller, PhD, grad of UC Berkeley.
Manufacturing
Arris (Berkeley, CA) – Advanced manufacturer with a breakthrough technology enabling the highest-performing fiber-reinforced composites at scale. Cofounder Erik Davidson has a Master’s degree in composites from UC Berkeley.
PhenomeX (Berkeley, CA) -Tools and instruments for the life sciences.
KoBold Metals (Berkeley, CA) – KoBold uses AI to search for signs of critical minerals. The strategy appears to have paid off: The company announced this year that it had discovered one of the largest copper deposits of all time, and it has raised nearly $500 million to exploit it. Cofounders Dr Kurt House has a BA from Claremont Colleges and a PhD from Harvard and Dr Josh Goldman has Ph.D. in physics from Harvard.
EarthGrid (Berkeley, CA) – The use plasma technology to make various types of tunnels for infrastructure needs. Founded by Troy Helming who is a grad of Univ Kansas and studied at U Penn.
Adams & Chittenden Scientific Glass Coop (Berkeley, CA) – Manufactures laboratory glassware and glass tools for scientific and industrial uses of all sorts. Their primary medium is borosilicate, or Pyrex glass. Their capabilities include the fabrication of standard laboratory glassware and all types of custom designed glass apparatus. They are manufacturers of OEM glass parts as well, in quantities of hundreds or thousands as required.
Limelight Steel (Oakland, CA) – Zero emissions steel production. Founded by Olivia Dippo, a UCSD grad and Andy Zhao, a Stanford grad.
Project Prometheus (San Francisco, CA) – Melding physics with AI to reinvent the physical world. Led by Dr. Vikram Bajaj, PhD, a grad of Univ Penn (BS), MIT (PhD) and UC Berkeley (Postdoc).
Eikon Therapeutics (Hayward, CA) – Eikon Therapeutics, cofounded by Prof. Dr. Eric Betzig, Ph.D., professor at UC Berkeley and Nobel Laureate for developing high-resolution microscopes, is a biopharmaceutical company that focuses on developing innovative technologies for drug discovery. The company utilizes advanced live-cell resolution microscopy and engineering to identify and create new therapeutic candidates. Eikon Therapeutics aims to enhance the understanding of biological processes at a cellular level, supporting targeted treatment development. The company’s approach integrates biology, engineering, and chemistry to streamline the drug discovery process. Through its proprietary platforms, Eikon Therapeutics seeks to advance clinical programs and expand its pipeline of potential medications. As of Jan 2026, Eikon Therapeutics has raised more than $1 billion and will soon launch an IPO. Another cofounder is Prof. Dr. Robert Tjian, Ph.D., a professor at UC Berkeley.
MicroFactory (San Francisco, CA) – Table-top AI-controlled manufacturing platform. Founded by Igor Kulakov who holds an MSc. in Electro-Mechanical Enclosure Engineering from Odessa National Polytechnic University and Viktor Petrenko a degree from South Ukrainian National Pedagogical University.
Velo3D (Freemont, CA) – Additive manufacturing technology using its own Laser Powder-Bed Fusion technology. Founded by Benny Buller, who has an MS from Technion Univ in Israel, and Erel Milshtein who has an MS from the Hebrew University.
Mainspring Energy (Menlo Park, CA) – Linear turbine technology that is flameless, low temperature reactions that are fuel efficient and less polluting. Currently deployed at Port of LA and Long Beach by Maersk. Founded by Dr. Shannon Miller, Ph.D., who has BS, MS, and PhD degrees in Mechanical Engineering from Stanford, where she was a National Science Foundation Fellow and received funding from the Global Climate Energy Project to advance her research. She was also recognized in 2012 by the MIT Technology Review as one of “35 Innovators Under 35. Dr. Matt Svrcek, PhD, who has BS, MS, and PhD degrees in Mechanical Engineering from Stanford; Dr. Adam Simpson, Ph.D., who has has a B.S. in mechanical engineering from Lafayette College and an M.S. and Ph.D. in mechanical engineering from Stanford University.
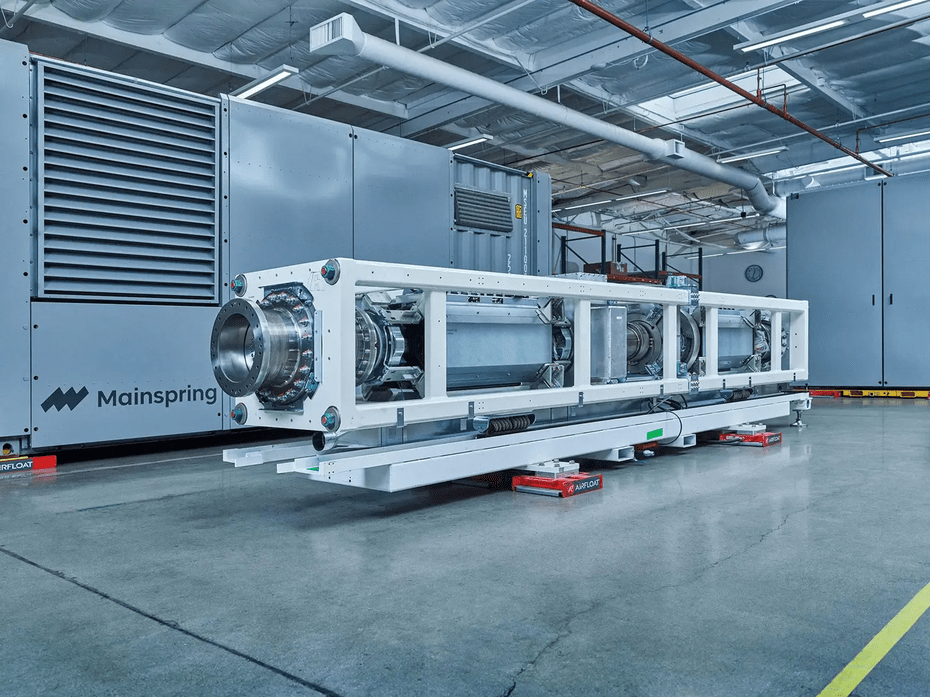
Linear turbine from Mainspring Energy in Menlo Park, CA
Artefact (Oakland, CA) – Green manufacturing materials and processes, in collaboration with UC Berkeley’s Berkeley Center for Green Chemistry.
Pow Bio (Berkeley, CA) – High efficiency fermentation platform for biomanufacturing. Founded by a UC Berkeley Ph.D. graduate and a Life Sciences executive, Dr. Ouwei Wang, PhD and Shannon Hall, BA from St. benedict and MBA from UC Berkeley.
Augmental Tech (San Francisco, CA) – Augmental Tech is a company that develops technology to help people with limited hand control use computers and mobile devices. The company was founded in 2019 by Tomás Vega and Corten Singer, both are UC Berkeley grads.
Resource (Oakland, CA) – Developed a process to make a bio-based platform chemical known as FDCA, which can be used to make plastics (such as PEF) that have better physical properties and superior performance over plastics made from fossil fuels. Founded by Aanindeeta Banerjee, PhD, a Stanford grad and Prof. Dr. Matt Kanan, PhD of Stanford.
Copper (Berkeley, CA) – They make induction stoves and ovens that are energy efficient and better performing tha gas stoves. Founder Dr. Sam Calisch has a PhD from MIT.
Elmworks (Berkeley, CA) – Next-generation electromagnetic devices. Developing additive manufacturing that can replace coil winding in the production of critical clean energy technologies like electric motors, wireless chargers, and next generation power converters. Founder Dr. Sam Calisch has a PhD from MIT and Tucker Gilman is a grad of Columbia University.
Akash Systems (Oakland, CA) – Oakland semiconductor startup Akash Systems was approved for more than $68 million in grants and tax credits through the CHIPS Act. Akash’s unique expertise in integrating synthetic diamond substrates with compound semiconductor materials like Gallium Nitride, the company utilizes its Diamond Cooling technology to improve thermal performance of semiconductors that need to maintain high-performance capabilities in challenging environments. This emerging technology, pioneered by Akash, is shown to improve heat dissipation of semiconductor devices, which strengthens performance and reliability in microelectronic systems. Founder and CEO Dr. Felix Ejeckam Ph.D is a grad of Cornell University in New York.
Artefact (Oakland, CA) – Materials & Manufacturing Tech, Reshoring US Manufacturing with Artefact Mfg Tech, Reducing carbon emissions by shifting manufacturing to the US, Replacing petroleum based materials with clean circular US agricultural materials.
Tensor Automotive (San Jose, CA) – Autonomous cars currently . Their care has 37 cameras, 5 lidars, 11 radars, 22 microphones, 10 ultrasonic sensors, 3 IMUs, GNSS, 16 collision detectors, 8 water-level detectors, 4 tire-pressure sensors, 1 smoke detector, and triple-channel 5G. Tensor is one of a few companies with a permit to test fully driverless vehicles on public roads in California. Founded in 2016 by former Princeton professor Dr. Jianxiong Xiao, Ph.D., a specialist in 3D learning, computer vision, and robotics.
Tarana Wireless (Milpitas, CA) – ngFWA is an entirely new technology built from the ground up to deliver reliable residential broadband. Overcoming multiple long-battled industry challenges, ngFWA provides affordable, fiber-class service with the deployment ease and scalability of wireless technology. Founded by Sergiu Nedevschi who was educated in Romania and UC Berkeley, and Dale Branlund who holds a Bachelor of Science in Electrical Engineering from California Polytechnic State University, and a Master of Science in Electrical Engineering from Santa Clara University.
Dynatomics (SF Bay Area, CA) – Dr. Larry Page, PhD, grad of Stanford has a new startup that is applying AI to product manufacturing.
Earth AI (San Mateo, CA and Stanmore, Australia) – AI software company focused on making predictions about potential mineral deposits, then approaching customers who might be interested in exploring sites further. Founded by Dr. Roman Teslyuk, PhD, a grad of the Univ of Sydney.
H2 Clipper (Santa Barbara, CA) – Specializing in hydrogen-based transportation and infrastructure, they have secured patents for a breakthrough in aerospace manufacturing using AI and swarm robotics. Founder Rinaldo Brutoco graduated from Santa Clara University in 1968 with a bachelor’s degree in economics and philosophy, and earned a Juris Doctor degree from the University of California, Los Angeles (UCLA) School of Law
SF Motors (Milpitas, CA) – SF Motors, Inc., dba SERES, is a customer-oriented E-Powertrain technology development & manufacturing company. Founder John Zang is a grad of Georgian College.
Lumina (San Francisco, CA) – Autonomous, electric tractors for industry and construction. Founded by Ahmed Shubber who has a degree from Fairfield University.
SnapMagic (San Francisco, CA) – AI Copilot for engineers who design electronics. Founder Jude Gomila has an MS from Cambridge Univ.
Acumen (Oakland, CA) – Green engineering. Founder Walter Allen, MS in engineering from UC Berkeley.
Sinovia Technologies (San Carlos, CA) – Printed organic light-emitting diodes (OLED). Founded by Dr. Whitney Gaynor, whose research at Stanford University focused on replacing the indium tin oxide transparent electrode in organic photovoltaics (OPV) cells. She has a BS in Materials Science from MIT and an MS and PhD in Materials Science from Stanford and Dr. George Burkhard who has a PhD from Stanford in optoengineering.
xMEMS (Santa Clara, CA) – Solid-State, All-Silicon Piezo MEMS Technology.
ReCarbon (Freemont, CA) – Converting carbon emissions into fuel. Founded by Dr Jay Kim, who has a PhD in physics.
The Hurd Company (Santa Monica, CA) – The Hurd Co makes agrilose: an agriwaste-based pulp used by apparel brands to make fabrics like viscose, rayon, and lyocell, which are normally made from trees. Agrilose is the same quality as standard wood pulp, and is available for the same price. Founder Taylor Heisly-Cook is a graduate of Northwestern University and the Bren School of Environmental Science and Management at UC Santa Barbara and David Mun received a Bachelor of Science degree from University of California, Los Angeles and a Master of Environmental Science from Bren School of Environmental Sciences at UCSB.
Felt (Oakland, CA) – They make maps with integrated datasets. Sam Hashemi and Can Duruk are grads of Carnegie-Mellon.
Other Lab (San Francisco, CA) – From solar powered motorcycles to off shore wind projects, this group is a research and design lab is led by Dr. Saul Griffth, Ph.D., a grad of MIT
Impulse Labs (San Francisco, CA) – Electric/battery appliances for the home. Founded by Sam D’Amico, a Stanford grad.
Pano (San Francisco, CA) Wildfire detection systems. Cofounded by Sonia Kastner, a physics grad of Harvard, MBA from Stanford.
SkySafe (San Diego, CA) – They build drone detection and warning systems. Founded by Grant Jordan a grad of UCSD.
Gridwrap (San Diego, CA) – Developed an advanced composite technology dubbed a Composite Wire Wrap that’s designed to mechanically reinforce existing aluminum conductor steel-reinforced (ACSR) cables. Adding composite material, such as carbon fiber, to high-voltage transmission lines allows for increased power capacity, which means that utilities can transmit more renewable energy to customers during high-demand hours
Pegbo (Menlo Park, CA) – They streamline supplier sourcing using its proprietary technology while supporting the participation of local, small, and diverse businesses in construction.
Firestorm Labs (San Diego, CA) – The build unmanned aerial systems.
Airbuild (San Diego, CA) – Climate technology company intended for carbon capture, water filtration, and solar energy generation. The company engages in producing sustainable materials to integrate carbon credit independence by turning their buildings into living breathing organisms that permanently sequester and detoxify water using algae while generating energy through embedded solar cells and sustainable materials, enabling companies and governments to sequester carbon, generate energy, and filter wastewater onsite. Founder David Gory has a degree in engineering.
Hadrian (Torrance, CA) – They produce parts, using precision robotics, to cheaply, quickly and efficiently that they can take over production and supply chain management for defense startups. founder and CEO is Chris Power, a graduate of Monash University in Australia.
Velo3D (Freemont, CA) – Velo3D manufactures the Sapphire range of metal powder bed fusion 3D printing systems, with the non-contact recoater often touted as a key benefit that increases the likelihood of optimal builds. Founded by Benny Buller, Bachelor’s and Master’s degree in Physics from Jerusalem University.
PTEC Soultions (Morgan Hill, CA) – Fiber optic systems engineering.
CTEMS (Freemont, CA) – Electromechanical engineering.
New Tech Solutions (Freemont, CA) – Building IT infrastructure.
Nextracker (Freemont, CA) – Solar tracking systems. Cofounder San Shugar has a BS in electrical engineering from Rensselaer Polytechnic Institute and an MBA from Golden Gate University, Alex Au a BS in engineering from UC Santa Barbara, and Marco Miller a degree from McGill University.
Machina Labs (Chatsworth, CA) – They take a sheet of material and turn it into a part in a manufacturing process they call Roboforming. AI driven robots form the parts, doing so fast, accurately, and in a repeatble fashion.
Bolt Threads (Emeryville, CA) – Founded by a doctor of chemistry from UCSF and doctor of biophysics from UC Berkeley, they make vegan leather and silk.
Air Protein (San Leandro, CA) – They make proteins out of the elements contained in air through their airFermentation process.
Algenesis (San Diego, CA) – A spin-out of UCSD, Prof. Dr. Stephen Mayfield, Ph.D., has developed a means to make plastic from plants. It’s sustainable and biodegradable. They have products in the market.
Picogrid (El Segundo, CA) – Picogrid builds a unified data integration system that connects fragmented sensors, cameras, and autonomous systems, backed by a suite of native hardware platforms. Founder Zane Mountcastle has a BS from NYU.
IrisVision (Pleasanton, CA) – Co-founded by Prof. Dr. Frank Werblin, Ph.D., at UC Berkeley (co-author and one of my mentors at Berkeley), they make augmented reality goggles for low-vision patients.
Astroforge (Huntington beach, CA) – They make machines to mine asteroids for precious metal and minerals. Cofounders Matt Gialich has an MS in engineering from Cal Poly, and Jose Acain has an MS in engineering from Santa Clara College.
Orbital Composites (Campbell, CA) – Robotic 3D printers enable the manufacturing of continuous fiber composites. With mastery of advanced materials and AI robotics, Orbital #Machines enable the manufacturing of humankind’s highest-performing composte structures. Cofounder Amolak Badesha earned a Bachelor of Science in Electrical Engineering from the University of California, Davis and a Master of Science in Electrical Engineering from Walden University
Cellibre (San Diego, CA) – Sustainable production of natural ingredients, including therapeutics and therpautic candidates. Supported by the NIH and Dept of Defense.
Onego Bio (San Diego, CA) – Sustainable, bio-identical egg protein. Supported by the DoD.
Savor Foods (San Jose, CA) – Sustainably produced fats. Supported by DoD.
Hadrian Automation (Torrance, CA) – Automated plants for manufacturing. A manufacturing process for a particular part is analyzed by Hadrian in collaboration with experts in the field, and then an automated manufacturing process, partially AI driven, is implemented in their factories.
Sift (El Segundo, CA) – Sift is empowers engineers with actionable data. Their platform ingests the high-velocity, high-cardinality data streams generated by complex hardware, transforming them into clear, contextual insights. With Sift, engineers can monitor the workings of their machines, proactively identify and resolve anomalies, and make data-driven decisions quickly.
Unspun (Oakland, CA)- The world’s first 3D weaving technology for apparel and the key to fashion’s waste problem. Their Vega™ 3D system weaves yarn directly into clothing, quickly and efficiently. Deployed in microfactories, Vega™ eliminates the need for large order quantities while reducing transport emissions and lead times. It’s a localized just-in-time system. They partner with brands and manufacturers who are committed to streamlining and decarbonizing fashion supply chains using automated, localized, and low-impact production. Cofounder Beth Esponnette has degrees from Cornell and Stanford, Kevin Martin is a grad of the Univ Colorado, and Walden Lam received a MBA from Stanford.
Aquam (San Diego) – We’ve all heard the Flint story – bad pipes contaominating the drinking water in Flint, Michigan. Aquam fixes old pipes of all kinds by perfusing the pipes with a coating that stops leaks, and the contamination of what flows in the pipes.
Limelight Steel (Oakland, CA) – Instead of burning biomass to make steel, they use LASERs to make zero-emissions steel. Cofounder Olivia Dippo is a grad of UC San Diego, and Dr. Andy Zhao, PhD has a doctorate from UCSD.
Resource Chemical (Oakland, Ca) – That biomass not used to make steel, can now be used to make clean plastics. ReSource has created technology to produce high-volume plastics that are made from truly sustainable feedstocks – including CO2 and inedible biomass – yet outperform conventional materials and can be easily recycled.
Skysdale (San Francisco, CA) – Smart ski poles that fold. Founded by Cristina Ashbaugh, a grad of Emerson College and Kelly McGee, a grad of MIT.
Range Energy (Mountain View, CA) – Electrifying the trailers on 18-wheel commercial trucks to extend the driving range of the rig.
Instrumental (Palo Alto, CA) – AI for manufacturing quality control. Founders Anna-Katrina Shedletsky is a grad of Stanford and Sam Weiss has a BS from MIT and a MS from Stanford.
Next Energy Technologies (Goleta, CA) – They make solar panels that also serve as windows. Founder Dr. Corey Hoven, PhD has a doctorate in materials science from UC Santa Barbara.
Ocean Technologies
HyperKelp (San Diego, CA) – Maritime intelligence systems, such as electronic buoys. Founded by Dr Graeme Rae, Ph.D. a grad of Ocean Engineering and Artificial Intelligence from Florida Atlantic University, and Costas Soler who has a BA in astrophysics from UC Berkeley and worked at NASA’s Space Sciences Laboratory at UC Berkeley.
AltaSea (Los Angeles, CA) – AltaSea at the Port of Los Angeles is dedicated to accelerating scientific collaboration, advancing an emerging Blue Economy through business innovation, and job creation, and inspiring the next generation, all for a more sustainable, just, and equitable world. They are currently deploying a mechanical device that converts wave action into electrical energy.
Splash Inc (Los Angeles, CA) – Developing the next generation of autonomous surface vessels (ASVs) to provide National Security and defend critical assets such as oil rigs and shipping terminals. Founder Ivan Avanesov has a BS from Univ. Pennsylvania and Marcell Veszpremi has EE degree from UCLA.
Robotics
Ambirobotics (Berkeley, CA) – Founded by Dr. Prof. Ken Goldberg, Ph.D. of UC Berkeley, they’ve commercialized AI robots for sorting materials in factories and distribution centers.
Covariant (Berkeley, CA) – Warehouse operations with AI robotic automation. The founders of Covariant AI are Prof Dr Pieter Abbeel, PhD of UC Berkeley, Dr. Peter Chen a PhD grad of UC Berkeley, Dr Rocky Duan, PhD from UC Berkeley, and Dr Tianhao Zhang, a PhD from UC Berkeley.
Kiwibot (Berkeley, CA) – Robotic delivery. I first saw this delivery system on the UC Berkeley campus delivering food to students. Founded by Felipe Chávez Cortés while a student at UC Berkeley.
Jacobi Robotics (Berkeley, CA) – They build software that makes robot arms faster and easier to program. Max Cao, Lars Berscheid and Yahav Avigal, launched Jacobi Robotics to build software that makes robot arms faster and easier to program. The three met at Berkeley AI Research Lab and started the company in 2022 based on their research in motion planning.
Robots.com (Berkeley, CA) – They have a number of different types of robots that are in service around the world, having completed more than 1.7 million tasks, the Company now powers delivery, logistics, and advertising robots for Fortune 500 customers across the United States, Canada, the United Arab Emirates and Saudi Arabia. Founded by Felipe Chavez, a grad of UC Berkeley.
Brain Corporation (San Diego, CA) – Their BrainOS® is the leading autonomy platform driving robotic and AI applications at scale, empowering organizations to automate and infuse intelligence into core operations with robots for factory and supply house applications. Backed by Qualcomm Ventures, the communications giant in San Diego that was cofounded by Prof. Dr. Irwin Jacobs, Ph.D., engineering professor at UCSD.
Squishy Robotics (Berkeley, CA) – Sensor robots that can be air-deployed into hazardous areas to furnish persistent, ground-level, real-time data for operations. Based on a tensegrity architecture. Cofounded by Dr. Alice Agogino, PhD, prof at UC Berkeley, Dr. Brian Cera, PhD and Dr. Deniz Dogruer, PhD, both engineering grads of Berkeley.
Covariant AI (Berkeley, CA) – Trained on the largest multimodal robotics dataset from warehouses around the world, the Covariant Brain enables robots to pick virtually any SKU or item on Day One. Founded by Prof. Dr. Pieter Abbeel, Ph.D., a professor of electrical engineering and computer science at the University of California, Berkeley, and his students, Peter Chen, Rocky Duan and Tianhao Zhang.
Physical Intelligence (San Francisco, CA) – Physical Intelligence is an AI company developing machine learning for robots and other physical devices. Currently valued at $2 billion for its progress toward creating software that can work for a variety of robots. In its latest funding round, PI raised $400 million from investors, including Jeff Bezos and OpenAI. Founded by UC Berkeley EECS professor, Prof. Dr. Sergey Levine, Ph.D, Prof. Dr. Chelsea Finn, PhD, a Stanford professor who attained her PhD at UC Berkeley.
1X Robotics (Palo Alto, CA) – 1X Technologies takes a practical approach to humanoid robotics, focusing on how robots can be useful in the home first, before anywhere else. Founded in 2014 as Halodi Robotics by Norwegian engineer Bernt Børnich, a grad of the Univ. Oslo, the company has grown from its Scandinavian roots into a global player. It now operates out of Palo Alto, with R&D hubs in Sunnyvale, California, and Moss, Norway.
Chef Robotics (San Francisco, CA) – Robotics for food preparation. Founded by Rajat Bhageria who has a master’s degree in robotics from U. Penn.
RIC Robots (Torrance, CA) – 3D printing robots for the building industry. Founder Ziyou Xu is a grad of Columbia University.

RIC Robots building a Walmart store.
Kodiak AI (Mountain View, CA) – Autonomous vehicles. Founder Don Burnette holds a Bachelor of Science degree in Computer Science from the University of California, Berkeley.
Field AI (Irvine, CA) – “Embodied AI” where AI is embodied into various physical forms of robots, running on their own without outside controls, such as GPS. Founded by Dr. Ali Agha, Ph.D., who is leading FieldAI’s strategic vision and product development. Prior to FieldAI, during his distinguished 7-year tenure at NASA’s Jet Propulsion Laboratory (JPL), Dr. Agha was Principal Investigator for some of the nation’s most high-profile and cutting-edge projects in autonomy, including the DARPA Subterranean Challenge, DARPA RACER (Self-driving off-road cars), NASA’s Autonomous Mars Cave Exploration, and Coordinated Autonomy for Prototype Mars Helicopter-Rover. Dr. Agha led the CoSTAR team (JPL-MIT-Caltech-KAIST-LTU), which won the Urban phase of 2020 DARPA Challenge focused on exploring unknown complex urban environments. Prior to JPL, Dr. Agha was a researcher at MIT and Qualcomm.
Bedrock Robotics (San Francisco, CA) – Robotic contruction machinery. Founded by Dr. Boris Sofman, PhD, grad of Carnegie Mellon, Kevin Peterson, MS from Carnegie Mellon, Tom Eliaz, a grad of UPenn, Dr. Ajay Gummalla, PhD, a grad of Georgia Tech.
Salidrone (Alameda, CA) – If you watched the videos of the ocean waves in the center of Hurricane Milton, you were viewing Salidrone’s seagoing robotic technology.
VenHub (Pasadena, CA) – Fully autonomous, robotic Smart Stores. Founded by Shahan Ohanessian, computer science grad of USC
Robust AI (San Carlos, CA) – Developer of autonomous mobile robots designed for warehouse and manufacturing operations. The company’s platform features AI-powered workflows and a human-centric design, enabling businesses to quickly deploy robots with minimal infrastructure changes, seamlessly scale operations, and support dynamic material handling tasks. Cofounded by Prof Dr Rodney Brooks, PhD of MIT (he now lives in San Francisco) and Prof Dr Gary Marcus, PhD of NYU, Anthony Jules, MSc from MIT, and Prof Dr Henrik Christensen, PhD, professor at UC San Diego.
Ekso Bionics (San Rafael, CA) – Exoskeleton technology for those with mobillity issues. Founded by Prof. Dr. Homayoon Kazerooni, Ph.D., a professor of Mechanical Engineering at the University of California, Berkeley, where he also serves as the director of the Berkeley Robotics and Human Engineering Laboratory.
Cyngn (Menlo Park, CA) – Autonomous DriveMod Tuggers, Forklifts and Stockchasers make intelligent, real-time decisions in factory settings. Cofounded by Ben Landen who has a MBA from UC Berkeley’s Haas School of Business, and BS Electrical Engineering at California Polytechnic University SLO.
Peanut Robotics (San Francisco, CA) – Capable and cost effective robots for the world’s labor market. Founder Joe Augenbraun has degrees from the Univ Delaware and gradute degree from Stanford, Ashis Gosh UC Berkeley College of Engineering and obtained a Master of Engineering degree in Product Design, Achille Verheye is a grad of Univ. Pennsylvania, and Stephen Hansen is a grad of UC Berkeley.
Tetsuwan Scientific (San Francisco, CA) – Autonomous technology firm building commercialization of artificial intelligence scientists specific to the life sciences. The company bridges the gap between lab robots and human scientists to breakthrough scientific discoveries, providing companies with artificial intelligence for research and development in the life sciences to achieve mass-manufactured scientific talent. Founded by Theo Schäfer who studied at MIT with a master’s in underwater autonomous robots and worked at NASA’s Jet Propulsion Lab exploring Jupiter’s moons for alien life and Cristian Ponce who graduated in bioengineering at Caltech.
Atom Limbs (Palo Alto, CA) – Mind controlled artificial limbs. Cofounder Tyler Hayes is a grad of St. Olaf College, Doug Satzger a grad of University of Cincinnati, and Eric Monsef has a Masters of Science degree in Engineering from Santa Clara University.
Serve Robots (Redwood City, CA) – Autonomous last mile delivery of small items. Founder Ali Kashani received both his Bachelor of Science in Computer Engineering and his Doctorate in Robotics from the University of British Columbia, Dimitri Demeshchuk received his Bachelor of Physics from Ulyanovsk State University, and MJ Chun received a Bachelor of Arts with High Honors from Swarthmore College.
Procept BioRobotics (San Jode, CA) – A surgical robotics company focused on advancing patient care by developing robotic solutions in urology. Founded by Dr. Nikolai Aljuri, Ph.D. who has a Dipl.-Ing. in electrical engineering from the Universität Fridericiana zu Karlsruhe (KIT) in Germany and holds a Ph.D. in Medical Physics from the Harvard-MIT Division of Health Science and Technology at the Massachusetts Institute of Technology (MIT).
Figure (Sunnyvale, CA) – Humanoid robots. Founded by Brad Adcock, BA Univ Florida. Their senior robotics engineer is Dr. Jenna Reher, PhD who attained her doctorate at Caltech.
Kind Humanoid (Palo Alto, CA) – Robotic company intended to optimize operations. The company specializes in delivering intelligent humanoids assisting people using large language models for high-level reasoning, enabling clients to automate and streamline tasks and operations. Cofounded by Dr. Christoph Kohstall, who has a PhD in physics.
Zoox (Foster City, CA) – This company actually has robotaxies operating, unlike that other company, Tesla, which operates on BS. Dr. Jesse Levinson, Ph.D. from Stanford, is the Co-Founder & CTO.
Rangeview (Berkeley and Los Angeles, CA) – The company has developed novel 3D printing technology for the production of molding casts and eliminating the need for support structures. The team at Rangeview has a strong background in robotics and are focused on reducing costs and increasing efficiency in the manufacturing process. Cofounded by Aeden Gasser-Brennan who attended UC Berkeley College of Engineering.
Canvas (San Francisco, CA) – They build robots for dry-wall construction, saving time and money. Currently operating in a number of projects, including the expansion of San Francisco International airport. Cofounded by Dr. Maria Telleria, Ph.D., graduate of MIT engineering, and Kevin Albert, MS in engineering from MIT. While visitng, stay at the best new hotel in the world, Luma Hotel, as ranked by Traveler’s Choice.
1X Technologies (Sunnyvale, CA) – Creating a large supply of labor through safe, intelligent robots. Founder Bernt Børnich has bachelor of robotics and nano-electronics from the University of Oslo.
Aurora (Mountain View, CA) – Self driving trucks and cars. Founder Dr. Drew Bagnall has a Ph. D. in Robotics from Carnegie Mellon, Dr. Sterling Anderson a PhD from MIT, and Dr. Chris Urmson a PhD from Carnegie Mellon.
Bonsai (San Jose, CA) – Agricultural and off-road robots.
Chef Robotics (San Francisco, CA) – Helps food companies by increasing production volume with flexible robotics and AI.
Cobot (Santa Clara, CA) -Robots for materials movement. Founder Brad Porter is a grad of MIT.

Food and Agriculture
Climax Foods (Berkeley, CA) – Led by a Ph.D astrophysicist from Berkeley Lab, Climax uses AI to develop healthy, vegan cheese. Their cheese is so good, it is currently used at Michelin-starred restaurants and has won numerous prestigious awards.

Pivot Bio (Berkeley, CA) – Crop nutrition technologies harness the power of nature to deliver nitrogen to plants. They enable microbes to convert atmospheric nitrogen and deliver it to crops, providing a source of nitrogen throughout the growing season. Cofounded by Dr. Alvin Tamsir, Ph.D., who earned a BS in molecular and cell biology from the University of California, Berkeley and a PhD at the University of California, San Francisco, and Dr. Karsten Temme, Ph.D., who earned BS and MS degrees in biomedical engineering from the University of Iowa and his PhD from the University of California, Berkeley.
Perfect Day (Berkeley, CA) – Uses the process of precision fermentation to create ProFerm™, a highly functional whey protein that contains no lactose, cholesterol, hormones, or pesticides. Cofounded by Dr. Bonney Oommen, Ph.D., who earned his PhD and MBA from Utah State University.
Novel Frams (Berkeley, CA) – Manufacture a variety of animal cells with specific attributes that influence flavor and nutrition profile.Theye use expertise in synthetic biology and cell biology to cultivate animal cells in the most efficient way possible to bring down the costs of animal cell manufacturing. Combined with data-driven multi-omic media formulation platform, they are able to cultivate an infinite spectrum of animal cells with tunable flavor and nutritional profiles. Cofounded by two UC Berkeley post-docs, Dr. Nieves Martinez Marshall, PhD and Dr. Michelle Lu, PhD.
Plonts (Oakland, CA) – Sustainable food products of different flavors and textures. The company offers a category of plant-based cheese items by fermentation and aging to turn mild milk into enchanting cheese with authentic tastes and varieties. Plonts was founded in 2019 by Dr. Nathaniel Chu, Ph.D. and Josh Moser. After completing his PhD at MIT studying the human gut microbiome, Chu wanted to apply his love for microbes to creating fermented foods from sustainable, nutritious, and affordable plants.
Upside Foods (Berkeley, CA) – Lab grown, sustainable meat. Cofounded by Dr. Bob Kiss, Ph.D., a UC Davis and MIT alum.

Cultivated chicken from Upside Foods.
Root Applied Sciences (Oakland, CA) – Root’s pathogen monitoring system can help growers manage their crop more effectively. Just as spraying when the pathogen is not there is wasteful, not spraying when the pathogen arrives can create a domino effect that allows the pathogen to flourish. Moreover, knowing when and where powdery mildew pressure is very high can help growers better manage an outbreak through identification and elimination of pathogen sources, leafing, and adjustments to the spray program. Founder Dr. Sara Placella, PhD has a BA in Earth and Planetary Sciences from Johns Hopkins University and a PhD from UC Berkeley in Environmental Science, Policy and Management where she focused on microbial ecology and soil biogeochemistry.
BlueNalu (San Diego, CA) – Lab grown seafood, replacing products that are inherently high in contaminants and that can be overfished, imported, or difficult to farm-raise. Founder Lou Cooperhouse received a MS in Food Science and BS in Microbiology, both from Rutgers University.
New Age Eats (Berkeley, CA) – Cultivated meat cells combined with plants to make meat products. Founder Brain Spears has a BS in chemical engineering from Brigham Young.
Wildtype (San Francisco, CA) – Cultured salmon. Dr. Aryé Elfenbein, Ph.D. and Justin Kolbeck co-founded Wildtype.
Finless Foods (Berkeley, CA) – Lab grown tuna. Cofounder Dr. Bryan Wyrwas, PhD is a molecular biologist and Michael Seldon has a degree in biochemistry.
California Cultured (Davis, CA) – Lab cultured coffee and chocolate. Cofounded by Alan Perlstein a grad of Columbia and an MS from NYU, and Dr. Harrison Yoon, PhD a doctorate in chemical engineering from Korea and a postdoc at Cornell.
Good Meat (Oakland, CA) – Sustainable lab grown meat. Founder Josh Tetrick was a Fulbright Scholar and grad of Univ Michigan.
HotSpot AG (Hanford, CA) – Automated solutions that empower farmers, water districts, and municipal agencies to maximize water efficiency while adapting to ever changing water conditions, policies and best practices. Founded by James Nichols, who has a degree in Crop Science and Management from UC Davis and continues to manage the farming operations at Nichols Farms while continuing to revolutionize the farming industry and establishing HotSpot AG as a leader in the field.
Bonsai Robotics (San Jose, CA) – Autonomous vehicles and their management for agriculture. Founders Tyler Niday has a degree from Cal Poly and a master’s from MIT, and Ugur Oezdemir has degrees from Tech Univ Berlin and Stanford.
Mission Barns (San Francisco, CA) – Cultivated meat. Various products on the market for those who eat meat. Mission Barns products contain real meat, without harming a single animal. A small sample from a pig is grown in a cultivator that mimics the animal’s body. Then, it’s combined with plant protein so you can enjoy Mission Barns meat. Founder Eitan Fisher has degress from Yale and Stanford.
The Better Meat Company (Sacramento, CA) – Mycoprotein-based meat. Founder Paul Shapiro is a grad of George Washington University.
Sensei Frams (Santa Monica, CA) – While the goal is good: using AI-powered greenhouses and robot harvesters to feed the world sustainably, the execution has been flawed. Led by egotistical people without proper education, Larry Elison and a physican named David Agus, the company has experienced too many problems like Wi-Fi issues and solar panels battered by Lanai’s winds — and rookie mistakes. Think greenhouses designed for Israel’s desert climate, when Lāna’i is typically muggy. The company also mixed mature and baby plants together, a blueprint for a pest paradise. Hopefully they’ll bring in some scientists to fix their mistakes and make of go of it.
Telesense (San Jose, CA) – Improve storage life and increase profitability with continuous remote monitoring of agricultural commodities. Cofounded by Prof. Dr. Naeem Zafar, PhD, a faculty member at the University of California, Berkeley,
Avela (San Diego, CA) – Manufacturer of R-1,3-butanediol a precursor to BHB-amino acid, BHB-Phe, can influence body weight and metabolism. Founded by Dr. Christophe Schilling, PhD, a grad of UCSD.
Zbiotics (San Francisco, CA) – Genetically engineered probiotics. Founded by Dr. Zack Abbott, PhD, has has degress from UC Berkeley and and Univ Michigan.
Solectrac (Windsor, CA) – Electric tractors. founded by Steve Heckeroth, who has an architecture degree from Arizona State.
Monarch Tractors (Livermore, CA) – Electric, autonomous tractors. Cofounded by Dr. Zachary Omohundro, PhD., alum of Carnegie Mellon University.
Farmwise (Salinas, CA) – Automated mechanical weeder that uses a combination of AI, computer vision and robotics to pull out weeds in vegetable fields without using chemicals. It has won several industry innovation awards related to agriculture and sustainability. Founded by Sebastien Boyer, a graduate of École Polytechnique and Massachusetts Institute of Technology, and Thomas Palomares, a graduate of École Polytechnique and Stanford University
Wild Genomics (San Diego, CA) – Pest control for crops. Founded by Drs. Eirik Torheim, PhD and Bilgenur Baloglu, PhD.
Farm Sense (Riverside, CA) – Pest detection to increase crop yields. Cofounded by 3 Ph.D. scientists.
Computers, Software, Semiconductors, and AI
Atom Computing (Berkeley, CA) – Atom Computing builds quantum computers using optically-trapped neutral atoms to create arrays of nuclear-spin qubits. They cool, trap, and control these qubits wirelessly using lasers. Their prototype platform, Phoenix, harnesses the power of 100 qubits to explore innovative quantum algorithms. They use next-generation systems with over 1,000 qubits that push the boundaries of system performance and scale.
Rigetti (Berkeley, CA) – Quantum processor chips are the foundation of their technology stack. Manufacturing these chips begins with the ability to design high quality quantum-coherent superconducting microwave devices. They leverage advanced modeling and simulation tools to design linear and nonlinear chip components, accurately predict performance behavior of large scale integrated quantum circuits (QuICs), and produce masksets to be fabbed in their own manufacturing facility, Fab-1. Founder Dr. Chad Rigetti, PhD, doctor of applied physics from Yale.
Fab-1 is a captive quantum integrated circuit foundry. They combine modern silicon semiconductor and MEMS processing technologies with novel manufacturing methods to produce state-of-the-art superconducting qubits and device layers for microwave circuitry. Their processes leverage superconducting materials such as aluminum, indium, and niobium in a series of subtractive patterning, etching, lithography, and deposition processes that result in ultra-low-loss superconducting devices.
Qolabs (Los Angeles, CA) – Building Quantum Supercomputers: Scaling from Hundreds to Millions of Qubits. Founded by UC Berkeley grad Prof. Dr. John Martinis, Ph.D., who is also a professor at UC Santa Barbara; Prof. Dr. Robert McDermott, Ph.D., a grad of UC Berkeley and prof at Univ Wisconsin; and Robert Ho, a grad of Univ. British Columbia.
Inversion Semiconductor (San Francisco, CA): Lithography systems for chip making using small particle accelerators. Founded by Rohan Karthik, masters degree in engineering from Imperial College, and Daniel Vega who has a MSc in Physics from University College London and a BSc in Applied Physics from UC Berkeley.
Unconventional AI (San Francisco and San Diego, CA) – energy-efficient AI hardware with its focus on fundamental AI compute and biology-inspired systems. Founded by Dr. Naveen Rao, Ph.D. whose doctorate is from Brown Univ.
PsiQuantum (Palo Alto, CA) – PsiQuantum’s thesis is that the unique strengths of photonic qubits, combined with direct leverage of high-volume semiconductor manufacturing, provide a fast, practical path to scale. Founded by Prof. Dr. Jeremy O’Brien, Ph.D. and Prof. Dr. Terry Rudolph, Ph.D.
Substrate (San Francisco, CA) – Supposedly making Advanced LASERs and Lithography systems for chip making using small particle accelerators. The company is led by a big tallking con-artist and is associated with Peter Thiel, a guy who’s had a string of failures that cost many people alot of money. They have investors, but none of the investors have expertise in semiconductors. On their web, they claim to be hiring a bunch of Ph.D’s with relevant experience. Will be interesting to see how this one plays-out.
xLight (Palo Alto, CA) – Advanced LASERs and Lithography systems for chip making using small particle accelerators. Founder Nicholas Kelez is a grad of UC Berkeley. The company is deep in talent with Dr. Chris Anderson, PhD, a grad of uc Berkeley; Dr. Andrew Burrill, PhD, a grad of State University of NY at Stony Brook; and Dr. Bruce Dunham, Ph.D., a grad of University of Illinois Urbana-Champaign – all have had senior level roles at national labs in photonics and particle acelerators.
Franz (Berkeley, CA) – Also known as AllegroGraph. Neurosymbolic AI. The complex scheduling of the James Webb Space Telescope and the Hubble Space Telescope is done using Franz’s underlying technology. Founded by a UC Berkeley PhD student in mathematics, Fritz Kunze, in 1984. Because Neurosymbolic AI is making a comeback given the shortcomings of current LLM models, I’ve included this seminal company.
Machine Learning Labs (San Francisco, CA) – An open source artificial intelligence research and product company. They’re building access to the knowledge and tools to make AI work for people’s unique needs and goals. Mira Murati is the CEO and a grad of Dartmouth, Andrew Tulloch is their Chief Architect nad he is a grad of Univ Sydney (BS), Cambridge Univ (MS) and working on a PhD at UC Berkeley, and Dr. John Schulman, PhD, a grad of UC Berkeley and the developer of the ChatGPT technology is the Chief Scientist. Unlike many of the AI bullshit artists, such as Sam Altman and Elon Musk, Murati and Schulman will tell you that AI is in its infancy.
Periodic Labs (Menlo Park, CA) – Has $300M in backing to develop robots that will run scientific experiments on a large scale. Founded by William Liam, a grad of MIT and Ekin Dogus Cubuk, a grad of Harvard.
Framework (San Francisco, CA) – Modular computers that allow easy upgrades by the owner. Founder Nirav Patel is a grad of Carnegie Mellon University.
Applied Intuition (Mountain View, CA) – Provides software infrastructure to safely develop, test, and deploy autonomous vehicles at scale. Founded by Mike Maples, BS engineering Stanford, and MBA from Harvard.
Symbolica AI (San Francisco, CA) – Neurosymbolic AI. Developer of a business model platform designed to help clients make structured reasoning. The company’s platform specializes in geometric and topological underpinnings of machine learning to train models using formal computational logic rather than statistical methods, enabling clients to be well-equipped to make business-enhancing decisions for their business growth.
Antimatter (Oakland, CA) – Data privacy solutions. a spinout of the RISELab at UC Berkeley, founded by two PhDs.
Motive (San Francisco, CA) – AI programs for business such as fleet management. Cofounded by Shoaib Makani, a grad of London Sch of Economics and Obaid Khan, a grad of Univ California, San Diego.
Atum works (Mountain View, CA) – Computer chip 3D lithography process to 3D print parts >10,000x cheaper in low-volume and 10x cheaper in mass-manufacturing compared to today’s 2D lithography. Founded by Lucas Pabarcius, a grad of Caltech and Stanford, and Malcolm Tisdale a grad of Caltech.
Harvey (San Francisco, CA) – AI analysis of legal documents. Founded by Winston Weinberg who holds a Bachelor of Arts from Kenyon College and a Juris Doctor from the University of Southern California Gould School of Law, and Dr. Gabe Pereyra, PhD, who earned a Bachelor’s degree in Computer Science from the University of Southern California and pursued a PhD in Neuroscience at the University of Oxford.
xAI (San Fancisco, CA) – AGI using LLM platform. Founded by Prof. Dr. Jimmy Ba, PhD, professor at U. Toronto and student of Prof Dr. Geof Hinton, PhD, prof at U. Toronto and former professor at UCSD.
Zip AI (San Francisco, CA) – AI business platform for product procurement and payment. Founded by Rujul Zaparde a grad of Harvard and Lu Cheng a grad of UC Berkeley.
Framework (San Francisco, CA) – They specialize in consumer electronics, computer hardware, and ethical tech. Bringing a RISC-V-based laptop to market in 2025. Founder Nirav Patel is a grad of Carnegie Mellon University.
Anysphere (San Francisco, CA) – Developer of an artificial intelligence-powered coding platform designed to help software engineers write code faster and more efficiently. The company automates common tasks in the software development process, such as linting, formatting, and code generation, enabling software engineers to focus more on creative and challenging aspects of their work. Founded by Michael Truell, Sualeh Asif, Arvid Lunnemark, and Aman Sanger, all of whom met while studying at MIT.
Hooglee (San Francisco, CA) – Their mission is to change the way people connect through the power of AI and video. Our team is creating innovative solutions that bring people closer, simplify communication, and enhance engagement. Cofounded by Dr. Eric Schmidt, PhD, a UC Berkeley grad.
Greaten (Berkeley, CA) – AI data mining of large, unstructured data sets. Founded by Dr. Harvey Li, Ph.D., who has a PhD in statistics and machine learning from UC Berkeley.
Gretel AI (San Francisco, CA) – Synthetic training data sets for AI development platforms. Founder Ali Golshan is a grad of Univ Calgary, Alexander Watson is a grad of Indiana Univ.
Epoch AI (San Jose, CA) – Epoch AI is a research institute investigating key trends and questions that will shape the future of AI. The Director is Dr. Jaime Sevilla, PhD who is a grad of the Univ Aberdeen.
Flock Freight (San Diego, CA) – AI-powered pooling technology for shipping optimizes across Flock’s entire customer network, typically combining 2-3 shipments from thousands of businesses into an efficient Shared Truckload. Founder Oren Zaslansky is a grad of Cal State Long Beach and has an MBA from Harvard.
Gatik (Mountain View, CA) – Autonomous trucks across networks, connecting regional distribution centers, warehouses, and local stores. They provide the AI service, not the truck. Founder Gataum Narang has an MS in robotics from Carnegie Mellon.
Ventana Mico Systems (Cupertino, CA) – RISC-V computer systems.
Recogni AI (San Jose, CA) – Developing its AI inference chip for both the generative AI and automotive industries. RK Anand has a MS from Syracuse Univ and Gilles Backhus has a MS from the Technical Univ of Munich.
Celestial AI (Santa Clara, CA) – Photonic-based integrated circuits. Founder David Lazovsky has a BS from Ohio Univ, and Preet Virk is a grad of Harvard.
Edge Cloud Link (Mountain View, CA) – Self powered data centers using hydrogen, and water production. Founder, CEO, Yuval Bashar is a grad of the Israel Institute of Technology.
Inception (Palo Alto, CA) – Diffusion large language models. Founded by Prof. Dr. Stefano Ermon, PhD, professor at Stanford; Prof Dr. Aditya Grover, PhD of UCLA who was a postdoc at UC Berkeley and PhD student at Stanford; and Prof Dr Volodymyr Kuleshov, PhD, a professor at Cornell and grad of Stanford.
Plaid Semiconductors (San Francisco, CA) – Plaid Semiconductors provides advanced interposers that surpass current market solutions, enabling heterogeneous systems with seamless integration of hundreds or thousands of chiplets. Theit technology is suited to next generation of AI and high-performance computing affording efficiency and scalability. Founder Dr. Pratik Nimbalkar, PhD is a grad of Georgia Tech
Ayar Labs (San Jose, CA) – Optical input/output computer technolgies. Cofounded by Dr. Mark Wade, Ph.D., a grad of MIT, UC Berkeley, and U. Colorado, and Dr. Vladimir Stojanovic, Ph.D. a professor at UC Berkeley and grad of Stanford.
Abundant AI (Freemont, CA) – When AI fails, as it often does, Abundant takes over. Its API allows it to catch when an AI agent fails, and allows one of its human operators to step in and take over. Fouinder Jesse HU is a grad of Duke, Meji Abidoye is a grad of U. Buckingham, Ke Huang has a BS and MS from Rice Univ.
SiFive (Santa Clara, CA) – Risc-V computer chips. Cofounded by Prof Dr. Krste Asanovic, PhD, professor at Berkelet EECS and Drs. Yunsup Lee, Ph.D. and Andrew Waterman, PhD, Berkeley EECS alums.
d-Matrix (Santa Clara, CA) – d-Matrix has focused on memory bandwidth and innovating on the memory-compute barrier. Thy’ve built a digital in-memory compute core where multiply-accumulate happens in memory and you can take advantage of very high bandwidth – about 150 terabytes per second. Founded by Seth Sheth and Sudeep Bhoja, both have MSEE from Purdue.
Nuvia (Santa Clara, CA) – Acquired by Qualcomm for over $1 billion, the company was founded by three engineers, Gerard Williams III, John Bruno, and Manu Gulati.
Snowflake (San Francisco, CA) – Snowflake moves back to San Francisco after moving to Montana and as a result losing ground to Data Bricks.
Cartesia AI (San Francisco, CA) – Multimodal intelligence tool designed to enable real-time, on-device artificial intelligence capabilities on every device. The company’s tool allows to facilitates a generative voice application programming interface, model architectures, and state space models across diverse modalities including text, audio, video, images, and time-series data, enabling developers to build sophisticated user interaction across various devices. Founders Dr. Karan Goel, PhD is a grad of Stanford, Dr. Albert Gu, PhD grad of Stanford, Dr Arjun Desai, PhD of Stanford, Brandon Yang grad of Stanford, and Dr Chris Ré, PhD of University of Washington.
Databricks (San Francisco, CA) – Data management with an AI component, a very successful billion dollar unicorn. Founded by Prof. Dr. Ion Stoica, PhD of UC Berkeley and his graduate students.
Anyscale (San Francisco, CA) – Anyscale is a configurable AI platform. Together, its components form a unified AI platform that gives your AI/ML builders tools to realize better production speed and cost efficiency. Developed at UC Berkeley by Prof Dr. Ion Stoica, PhD and his students at Berkeley. Currently used by many companies, including OpenAI.
Tenstorrent (Santa Clara, CA) – Tenstorrent develops advanced AI hardware and software solutions for data processing and machine learning application. Recently moved from Toronto to California.
NinjaTech AI (Los Altos, CA) – Cross-platform AI agents. Founded by Babeck Pahlavan who has a M.S. in Electrical Engineering from Stanford University, and a B.S. in Electrical Engineering and Computer Sciences (EECS) from UC Berkeley, Sam Naghshineh who has a BS in engineering from U. Arizona, and Dr. Arash Sadrieh, PhD whose doctorate is from Oxford in computer simulations.
Sandbox AQ (Palo Alto, CA) – Large Quantitative Models (LQMs) for life sciences, financial services, navigation, cyber and other sectors.
Blue Qubit (Los Angeles, CA) – Developer of a quantum computing platform designed to create a universal software library for quantum computers. The company provides users with operating systems to run specifically on gate-based quantum algorithms, and provides job metadata and history through software manufacturing and quantum computing. Cofounder Dr. Hrant Gharibyan, PhD is a grad of Stanford.
PsiQuantum (Palo Alto, CA) – Using light in silicon chips to create a giant programmable quantum computer that can outperform classical machines — and do it soon. By the end of 2027. They use light as a qubit. Their “photonic” quantum computing is now claimed in 2025 to commercially scalable. Cofounded by Prof. Dr Jeremy O’Brien, PhD of Stanford, Prof. Dr. Terry Rudolph, PhD of Imperial College, Dr. Pete Shadbolt, Ph.D, and Dr. Mark Thompson, Ph.D.
HuLoop AI (Auburn, CA) – Automation for businesses. Seamlessly automates business processes spanning across diverse systems (web, mobile, desktop, packaged apps, and data sources).
Silicon Catalyst (Palo Alto, CA) – The only incubator + accelerator focused on the Global Semiconductor Industry including Chips, Chiplets, Materials, IP and Silicon fabrication based Photonics, MEMS, Sensors, Life Science and Quantum.
QC Ware (Palo Alto, CA) – Quantum-computing-as-a-service company. Founders Matt Johnson, MBA (UPenn, Wharton) BS (US Air Force Academy); KJ Sham, MBA, PhD (Electrical Engineering, University of Minnesota), M.Eng (EECS, MIT).
Astera Labs (Santa Clara, CA) – Chips and software for AI. Founder Jitendra Mohan has an MSEE from Stanford University, Sanjay Gijendra has a master’s degree in engineering management from University of Colorado at Boulder, and Casey Morrison an MSEE from the University of Florida.
SiMa (San Jose, CA) – Chips for edge AI. Founded by Krishna Rangasayee, who has an MS in engineering from Mississippi State.
ReconRF (San Diego, CA) – Manufacturer of monolithic microwave integrated circuits designed to distribute circuit and electromagnetic simulations across multiple processors. The company leverages parallel computing to readily optimize on-chip component values with EM-level accuracy to explore design options and verify the performance of large-scale systems, enabling customers to use the distributed EM analysis option in cadence RF technologies to accelerate product development and enhance amplifier performance.
Mobix Labs (Irvine, CA) – Mobix Labs delivers next-generation connectivity solutions that address the toughest challenges in 5G wireless and advanced communications systems, including space systems. Founder Fabian Battaglia has a BSEE from Lawrence Technological University in Southfield Michigan.
Perplexity AI (San Francisco, CA) – Conversational search AI model that is designed to answer queries using natural language predictive text. It generates answers for users using web-based sources, but also boasts the unique function of citing the webpage links from it is generating its answers. Cofounded by Dr. Arind Srinivas, Ph.D., doctorate awarded from UC Berkeley, Dr. Andy Konwinski, PhD, doctorate from UC Berkeley, Dr Dennis Yarats, PhD, doctorate from NYU.
Ava (San Francisco, CA) – Enabling deaf and hard-of-hearing people and inclusive organizations with the best live captioning solution for any situation. Part of UC Berkeley’s SkyDeck Incubator.
Launch Darkly (Oakland, CA) – Product launch platform. Founder Edith Harbaugh has a BS engineering degree from Harvey Mudd and an MS in economics from Pomona College, both presitgious liberal arts colleges located near Los Angeles.
11X ai (San Francisco, CA) – Digital workers for Sales, RevOps, and Go-to-Market Teams. Another company that moved to California.
Krea AI (San Francisco, CA) – Another AI company that moved from Florida to California.
Notion AI (San Francisco, CA) – Productivity software. Cofounded by Ivan Zhao, a grad of U. British Columbia, and Chris Puchua, a grad of U. Michigan.
Anthropic AI (San Francisco, CA) – LLMs with a focus on safety. Cofounded by Univ Claifornia Santa Cruz alum, Daniela Amodei who graduated summa cum laude from UCSC with a Bachelor of Arts in English Literatureand Dr. Dario Amodei, Ph.D., an alum of CalTech, Stanford, and Princeton.
Letta (San Francisco, CA) – Developing LLMs as operating systems. Founded by Berkeley PhD students Sarah Wooders and Charles Packer at Berkeley’s SkyDeck, with their advisor, Prof. Dr. Ion Stoica, Ph.D., EECS professor at Berkeley.
SandboxAQ (Palo Alto, CA) – AI quantum computing, developing Large Quantitative Models (LQMs), address key challenges in drug discovery, new materials for the auto and aerospace sectors, and investment asset management. Founded by Dr. Eric Schmidt, PhD, a UC Berkeley alum, and Jack Hidary a graduate of Columbia University.
Abel (San Francisco, CA) – AI for identifying criminals, limiting misidentification of the innocent.
Personal AI (San Diego, CA) – They build small language model AIs for enterprises handling sensitive data and proprietary information that can’t be uploaded to models like ChatGPT. Led by CEO Suman Kanuganti, a UCSD Rady School of Management MBA graduate, Personal.ai has already made significant strides since launching its product 10 months ago, boasting nearly $2 million in annual recurring revenue
Single Sprout (Newport Beach, CA) – Another AI company that moved to California.
Open AI (San Francisco, CA) – Large language models for research and business applications. Cofounded by Dr. John Schulman, Ph.D., Berkeley Ph.D. alum from EECS. Just closed one of the largest fundraising rounds in U.S. history. A $6.6 billion recent raise is a huge sum that will fuel its cash-burning work of researching and selling AI products. The fresh cash gives OpenAI a paper valuation of $157 billion.
World Labs (San Francisco, CA) – Led by Prof. Dr. Fei-Fei Li, Ph.D., the Stanford professor many deem the “Godmother of AI,” has raised $230 million for her new startup from backers including Andreessen Horowitz, NEA, and Radical Ventures. Dr. Li did her Ph.D. at CalTech with Dr Pietro Perona, Ph.D, a Berkeley Ph.D. alum. Cofounder is Dr. Ben Mildenhall, Ph.D, a Ph.D alum from UC Berkeley. You see how Berkeley, CalTech, and Stanford are seminal in AI. WL is developing what it calls “large world 3D spatial models” that will be used by professionals such as artists, designers, developers, and engineers.
Micropsi (San Francisco, CA) – Another company that’s moved it’s HQ to California. They do AI vision for robotic applications.
Fire Aside (San Anselmo, CA) – Fire Aside offers a suite of products to fire departments and other public safety agencies to digitize inspections of homes and businesses for compliance with defensible space and home hardening requirements.
Cloudflare (San Francisco, CA) – AI to protect your website from being scraped by unwanted bots. Their technology derives from the patented work of Prof. Dr. Arthur Keller, Ph.D. at Univ. California Santa Cruz, who attained his Ph.D. at Stanford.
Groq AI (Mountain View, CA) – AI chips and software founded by a group of former Google engineers. Groq LPU executes complex neural network operations in a highly parallelized manner, 18X faster than normal GPU architectures. Cofounded by Jonathon Ross, a graduate of NYU in mathemtics and CS.
Cerebras (Sunnyvale, CA) – Huge, fast chips and cloud supercomputers for generative AI. They’re in the market and challenging Nividia. Their team is loaded with talent, including MS and PhD engineers from Switzerland, Stanford, UCLA, and Berkeley, including Michael James, a UC Berkeley alum.
Memcomputing (San Diego, CA) – Connected computational units that dynamically respond to one another with integrated memory. A spinout of UCSD professors.
Keneron (San Diego, CA) – On-edge AI chips. Founded by Dr. Albert Liu, PhD who graduated from a joint UCLA/Berkeley PhD program.
SambaNova (Palo Alto, CA) – Configurable architecture helps developers squeeze efficiency from AI models. Reconfigurable dataflow architecture composed of tiles of memory and compute resources. The links between these tiles can be altered on the fly to facilitate the quick movement of data for large neural networks. Led by Rodrigo Liang, who has BS and MS degrees in EE from Stanford, and much industry experience.
Rain Neuromorphics (San Francisco, CA) – building computer chips based on the brain’s architecture. Like the brain, Rain circuitry uses both analogue and digital processing. Cofounded by Dr. Juan Claudio Nino, PhD, alum of Penn State.
Valar Labs (Palo Alto, CA) – AI for detection of solid tumors. Founded by Anirudh Joshi, BS from Georgia Tech and MS from Stanford.
Etched AI (San Francisco, CA) – AI transformer chips that are much faster and less expensive than GPU chips, Cofounder Chris Zhu graduated Summa Cum Laude from Harvard University.
Safe Superintelligence Inc. (Palo Alto, CA) – They’ve raised $1 billion in a seed round! Stay tuned. Talk about hype, Safe Superintelligence’s valuation ballooned from $5 billion to $30 billion since its launch last June, 2024. Founded by Dr. Ilya Sutskever, PhD, a student of Prof Dr. Geoffrey Hinton, PhD at Univ Toronto.
pSemi (San Diego, CA) – integrated semiconductor devices.
Ubilite (San Diego, CA) – Wi-fi chips. Cofounded by Dr. Peter Gammell, Ph.D., alum of MIT and Cornell.
Obsidian Sensors (San Diego, CA) – Thermal sensors, founded by John Hong, alum of MIT.
Yembo AI (San Diego, CA) – AI for moving. Founded by 2 engineers formerly of San Diego tech giant, Qualcomm.
LeapFrog Semiconductor (San Diego, CA) – Semiconductors for 5G.
Elevate Semiconductor (San Diego, CA) – Semiconductor testing systems. Founded by Patrick Sullivan holds a Bachelors degree in Electrical Engineering from The University of Wisconsin
Lightmatter (Mountain View, CA) – With the end of Moore’s Law, scaling AI performance at the chip level increasingly requires integrating more silicon in a single package. Many GPU and accelerator providers address this by incorporating multiple processor, memory and I/O chiplets onto an electrical silicon interposer. While this approach boosts compute capabilities within a package, the I/O bandwidth of the chip is severely constrained by the limited shoreline and the competing need to integrate more memory. Lightmatter’s Passage platform overcomes these shoreline constraints by using 3D integration of customer dies directly onto a silicon photonic interconnect, enabling optical I/O anywhere across the chip area. This platform provides significantly higher connection density and bandwidth both within and outside the package. Additionally, it natively integrates Optical Circuit Switching (OCS) within the interconnect, offering enhanced resiliency and flexibility in interconnect topology.
Founded by Dr. Nicholas Harris, PhD, doctorate in Electrical Engineering and Computer Science at the Massachusetts Institute of Technology; Dr. Darius Bunadar, PhD, obtained his PhD in physics at MIT studying quantum computation and communication using compact nanophotonic circuits; Thomas Graham, grad of Georgetown and MBA from MIT.
Ampere (Santa Clara, CA) – Develops processors for servers operating in large scale environments. Founded by Renee James, BA and MBA from U. Oregon.
Fundamental Research Labs (Menlo Park, CA) – Develops apps that allow you to chat with an AI bot, connect applications, and ask questions across the knowledge bases of those applications, then ask it to schedule appointments for you on your calendar. The app can schedule workflows for you to repeatedly execute some tasks. The apps allow startup engineers to test out various capabilities of models and platform tech it is developing. Founder, Dr. Robert Yang, PhD, is a professor at MIT,
Green Energy
Twelve (Berkeley, CA) – Twelve is converting carbon dioxide emissions into sustainable jet fuel. It recently launched the first commercial-scale production facility for power-to-liquid sustainable aviation fuels in the US. They’ve garnered huge amounts of investment from companies like Microsoft, Shopify, and Alaska Airlines—which see Twelve’s technology as key to reaching their own sustainability goals—are underwriting some production costs and may help the company scale. Cofounder Dr. Kendra Kuhl, Ph.D., has a doctorate in chemistry from Stanford and Nicholas Flanders has a M.S. in engineering and MBA from Stanford University.
Harvest Thermal (Berkeley, CA) – Efficient heat pump and a “smart thermal battery” to heat, cool, and provide hot water in homes, all using clean energy. Cofounded by Dr. Jane Melia, Ph.D. and her husband who is an engineer.
ARCHES h2 (Oakland, CA) – A corporation composed of a consotium of groups to bring green energy to California, especially hydrogen.
Terrebase Energy (Berkeley, CA) – Terabase is building an interconnected digital and automation solar platform that reduces cost and increases scalability. Founded by Matt Campbell, BA degree from the University of Wisconsin and an MBA from UC Berkeley.
Deep Fision (Berkeley, CA) – Underground small modular reactors (SMRs). Cofounded by Dr. Richard Muller, PhD, doctorate in physicis from UC Berkeley and Elizabeth Muller a grad in mathematics from UC San Diego and an MBA from Paris.
Calectra (Oakland, CA) – Thermal energy storage systems. The technology involves using bricks to convert electricity into high-temperature heat, then storing and delivering that heat — potentially up to 1,600 degrees Celsius — by piping it to industrial manufacturers. Founder Pauliina Meskanen has BS and MS degrees in engineering, and Dr. Nate Weger, PhD is an engineering grad of UC Berkeley.
Marathon Fusion (San Francisco, CA) – Nuclear fusion for energy. Recently, Marathon turned its attention to nuclear transmutation, proposing to introduce a mercury isotope, mercury-198, into a fusion reactor to turn it into mercury-197, and they also plan to produce gold. Founded by Adam Rutkowski who has a Bachelor of Arts in physics at Carleton College and his Master of Arts in astrophysical sciences at Princeton University.
Pacific Fusion (Freemont, CA) – Dr. Keith LeChien, Ph.D., Pacific Fusion co-founder and chief technology officer, invented a technology that more than doubles the efficiency and power density of what Sandia researchers did with the Z Machine. Dr. LeChien is a grad of Univ Missouri.
Intersect Power (San Francisco, CA) – Intersect has grown exponentially as it moves forward in building AI data centers with a “power-first” approach that co-locates data centers with power generation, using a combination of wind, solar, battery storage, and flexible gas generation Founded by Sheldon Kimber who earned a BA from Kenyon College and an MBA from UC Berkeley Haas School of Business.
Fuse Energy Technology (San Leandro, CA) – Their fusion reactor TITAN is different from other fusion contraptions that use large doughnut-shaped units called tokamaks that confine superhot plasma with magnetic fields. TITAN is a magnetized target machine, creating powerful, instantaneous electrical energy to rapidly compress and heat plasma, achieving the ultra-high temperature and pressure required for fusion. Founded by JC Btaiche, who was born in Lebanon to a nuclear physicist.

Biogenic Energy (San Francisco, CA) – Green energy from hydrogen. Founded by Prof. Dr. R. W. Dibble, Ph.D., who is the head of the Combustion Laboratory at the University of California, Berkeley; Dave Williamson who has a BS in Civil Engineering from Tulane University in New Orleans; Craig Mlott who has a BS in Electrical Engineering and BA in Economics from Stanford University and a MBA from the Anderson School of Business at UCLA.
Aetherflux (San Carlos, CA) – Beaming solar power from space. Founded by Baiju Bhatt who has an MS from Stanford.
SunTrain (San Francisco, CA) – Manufacturer of reliable renewable energy storage intended to provide affordable energy storage to consumers. The company makes decarbonization possible and profitable by leveraging existing infrastructure and technologies and delivering renewable energy using existing rail resources, enabling clients to access on-demand and affordable storage anywhere.
Verne (San Francisco, CA) – Hydrogen storage and transportation. Founded by Dr. David Jaramillo, PhD who has a PhD in chemistry from UC Berkeley, Ted McKleeven, a BA from Harvard and MBA from Stanford, and Bav Roy has an engineering degree from UNSW and an MBA from Stanford.
Antares Nuclear (Redondo Beach, CA) – Fission microreactors. Founder John Bramble is a grad of George Mason Univ and Julia Dewahl is a grad of Dartmouth.
Zitara (San Francisco, CA) – Clean energy management software. Founders Shyam Srinivasan and Evan Murphy both hold a BS in Electrical Engineering from Caltech.
AltaSea (Los Angeles, CA) – A public-private ocean institute that convenes and nurtures the best and brightest pioneers and organizations in science, business, and education.
Specifix (Los Angeles, CA) – HVAC data management for optimized performance. Founded by Ryan Martineau, a UC Berkeley grad, Pete Shimkus, an ASU grad, and Ben Patch, a grad of Dartmouth.
Electric Hydrogen (San Carlos, CA and Boston, MA) – Hydrogen electrolyzers.
Equatic (Los Angeles, CA) – Created from more than a decade of research and development at UCLA’s Samueli School of Engineering, the patented technology accelerates the ocean’s inherent ability to absorb and permanently store massive amounts of carbon while simultaneously producing carbon-negative hydrogen. Founded by Dr. Gaurav N. Sant, Ph.D., Pritzker Professor of Sustainability in UCLA’s Samueli School of Engineering, where he is also the Founder and Director of UCLA’s Institute for Carbon Management.
Bloom Energy (San Jose, CA) – Clean energy platform, including hydrogen. Founded by Dr. KR Sridhar, PhD, who has a M.S. in nuclear engineering and a Ph.D. in mechanical engineering from University of Illinois at Urbana-Champaign
EnviroGenTechH2 (Sacramento, CA) – Non-mechanical system is low pressure system: < 100 psi. Process is also low voltage, helping mitigate volatility risk of mechanical H2 production. E-Catalyst (e-Cat) fuel can be formulated in plate, rod and pellets delivery formats and only needs the introduction of a soluble source such as water to provide truly inert energy production. 100% Eco-Friendly and completely benign after use. e-Cat fuel can be easily recycled or disposed of because it contains no harmful elements. Comparable energy density to diesel fuel
Capstone Green Energy (Van Nuys, CA) – Green energy gas turbine manufacturer that specializes in microturbine power along with heating and cooling cogeneration systems.
Ionic Power (Sacramento, CA) – Solar-powered mobile workspaces combine the comforts of home with cutting-edge clean energy, battery backup, and durable design.
General Galactic (El Segundo, CA) – Carbon capture for energy production. Founders Halen Mattison and Luke Neise has MS degrees from Stanford, and Michael Adeyi a BS in chemical engineering from Yale.
Fuse Energy Technologies (Palo Alto, CA) – Fusion energy.
Zum (Carson, CA) – Integrated green transportation systems for schools. Founded by Ritu Narayan, a grad of Stanford.
GKN Hydrogen (Carlsbad, CA) – GKN Hydrogen designs, manufactures, markets, and sells energy storage and hydrogen storage system utilizing metal hydride technology that help organizations decarbonize operations across a range of industries.
Atoco (Irvine, CA) – CO2 capture using COFs and MOFs (metal-organic frameworks), both of which are rigid crystalline structures with regularly spaced internal pores that provide a large surface area for gases to stick or adsorb. Developed by Prof. Dr. Omar Yaghi, Ph.D., professor of chemistry at UC Berkeley. Just half a pound of the powder may remove as much carbon dioxide as a tree can, according to early tests. This is a potential game changer for carbon removal.
TAE (Irvine, CA) – Founded by a UC Irvine professor, Prof. Dr. Norman Rostoker, Ph.D., they’re in the race to create a commercial fusion reactor. Dr. Michl Binderbauer, Ph.D., is their CEO. TAE uses a unique field-reversed configuration. After two plasma shots collide in the middle of the reactor, the company bombards the plasma with particle beams to keep it spinning in a cigar shape. That improves the stability of the plasma, allowing more time for fusion to occur and for more heat to be extracted to spin a turbine. TAE has raised $1.32 billion.
Alpha Ring (Monterey, CA) – Alpha Ring’s method, considered by industry leaders to be unconventional, is known as “electron-catalyzed fusion.” The process relies on what the company describes as “miniaturized” nuclear reactors, which are designed to be small enough to fit on a tabletop, but large enough to power entire communities. Their CSO is Prof. Dr. Roger Falcone, Ph.D., a physics professor at UC Berkeley.
Pacific Fusion (Freemont, CA) – Will Regan, a graduate of UC Berkeley is their CEO. Founded in 2023, they are operating in stealth and hiring many high level physicists and engineers at this time.
Aetherflux (San Carlos, CA) – Solar arrays in space that deliver energy to Earth. Founded by Baiju Bhatt who earned his BS and MS degrees at Stanford.
Terraform Energies (Burbank, CA) – Converts electricity and air into synthetic natural gas. Founder Dr. Casey Handmer, PhD is a grad of Caltech.
Xcimer (Denver, CO and Redwood City, CA) – Xcimer uses a straightforward approach: follow the basic science that’s behind the National Ignition Facility’s breakthrough net-positive experiment, and redesign the technology that underpins it from the ground up. Their aiming for a 10-megajoule laser system, five times more powerful than NIF’s setup that made history. Molten salt walls surround the reaction chamber, absorbing heat and protecting the first solid wall from damage.
Marathon Fusion (San Francisco, CA) – building fuel processing technology to make fusion power plants robust, practical and widespread.
Harvest Thermal (Kensington, CA) – low energy heat pumps. Cofounded by Dr. Jane Melia, Ph.D., a Cambridge PhD in fluid dynamics. They’ve won many awards for innovation and sustainability.
RedoxBlox (San Diego, CA) – Thermochemical energy storage (TCES) units store energy both chemically and as heat at very high temperatures that can be discharged continuously or as needed in place of burning fossil fuels. The TCES system has higher energy density than lithium-ion, at a lower cost. Founded by Prof. Dr. James Klausner, PhD, a professor at Michigan State, and Prof. Dr. Jörg Petrasch, PhD, also a professor at Michigan State.
Arbor Energy (El Segundo, CA) – Arbor is developing an innovative engine that uses advanced space technology to generate clean electricity while permanently removing CO2 from the atmosphere.
Radiant Nuclear (El Segundo, CA) – Portable, micro-nuclear reactors for energy. Cofounder Bob Urberger graduated from Saint Louis University with a BS in Computer Engineering
Stax Engineering (Oakland, CA) – They build green barges that serve as giant vacuum cleaners, capturing exhaust from container ships while they’re berthed at port. Founder Mike Walker attaended UC Berkeley and is a grad of USC.
Nuvve (San Diego, CA) – Patented vehicle-to-grid electrification. Founded by Greg Poilasne who has an MBA from the Wharton School of Business, University of Pennsylvania, a Ph. D. in Electrical Engineering from the University of Rennes.
Nextracker (Freemont, CA) – They make solar trackers, advanced systems that follow the sun’s movement throughout the day to ensure maximum energy generation. Founded by Dan Sugar who has BS in electrical engineering from Rensselaer Polytechnic Institute and an MBA from Golden Gate University, Alex Au is a grad of California Polytechnic State University-San Luis Obispo, Marco Miller is a grad of McGill University in Montreal, Canada, Mike Mahawich holds an MBA from the University of Chicago and a BS in Industrial Engineering from Northwestern University, and Marco Garcia has dual degrees in Electrical Engineering and Economics from Brown University.
Graphatic Energy (Santa Barbara, CA) – Graphitic Energy’s technology breaks hydrocarbons into clean hydrogen and solid carbon—making the carbon in natural gas an asset instead of a liability. Founder Zack Jones is a grad of Duke University.
Valar Atomics (Hawthorne, CA) – Scaling nuclear energy for heavy industrial power and clean hydrocarbon fuel production. Their reactor uses high-temperature gas reactor (HTGR) design principles with an improved safety profile and proliferation resistance when paired with TRISO fuel.
Peak Energy (Burlingame, CA) – Veritically integrated battery storage systems, including sodium-ion batteries. Founders Moss Landberg is a grad of Wake Forest, cameron Dales is a grad of Stanford, and Liam O’Connor has a master’s degree from U. Penn.
Aerospace
Astro Mechanica (San Francisco) – They’ve invented a new kind of jet engine – the Electric Adaptive Engine. Unlike any existing engine, it’s efficient at every speed. Because it’s efficient at every speed, it enables them to build a new jet aircraft with unique capabilities. It can launch payloads to orbit for 3x cheaper or fly 3x faster than regular passenger aircraft. Compressor is independent of the turbine, each of which can run at their own optimal speed. Founded by Ian Brooke who has physics degree from U. Colorado and masters degree from San Francisco State in engineering. Well described tech in an S3 program.
Astronis (San Francisco, CA) – They build commercial satellites in downtown San Francisco. Their technology uses geostationary satellites to provide widespread communications across the globe. This is far superior in performance to the low earth orbit satellite technology being used by starlink that crash to Earth in four years and destroy our atmosphere. Founder John Gedmark earned a BS from Purdue University and a Master of Science degree from Stanford University, both in Aerospace Engineering, with a focus on rocket propulsion.
Pyka (Oakland, CA) – Autonomous aircraft that are currently being used as cropdusters throughout the world. Cofounder Michael Norcia has a BS in physics from UC Davis.

Astro Digital (Santa Clara, CA) – Integrated satellite platforms. Founded by Chris Biddy, who has an M.S. in engineering from California Polytechnic University.
Apex (Los Angeles, CA) – Apex manufactures smallsat buses for commercial and government customers. They recently raised $200 million in a new funding round intended to help the company accelerate production of satellite buses. Founder Ian Cinnamon has a BS from MIT and a MBA from Stanford.
Rocket Lab (Long Beach, CA) – Rocket Lab is an end-to-end space company delivering launch services, complete spacecraft design and manufacturing, satellite components,
flight software.
Helicity Space (Pasadena, CA) – Helicity Drive, consists of scalable, fusion propulsion engines that enable safer, faster, reusable, and more fuel-efficient travel into deep space. Cofounder Dr. Setthivoine You, PhD, Associate Professor at The University of Tokyo and an Assistant Professor in Aeronautics & Astronautics at the University of Washington, Dr. Stephane Lintner, PhD, a grad of Caltech, and and Marta Calvo has a Master of Business Administration from the Bordeaux School of Management in France.
Supernal Aero (Irvine, CA) – Developing an electric vertical take-off and landing (eVTOL) vehicle and a scalable, electrified, clean-energy ecosystem that will integrate seamlessly with existing transit infrastructure. Currently being tested in California’s Mojave Desert. Founded by Dr. Jaiwon Shin, PhD, an engineering grad of Virginia Polytechnic Institute.
Inversion Space (Los Angeles, CA) – Space rentry vehicles. Inversion closed a $44 million Series A round in 2024 co-led by Spark Capital and Adjacent, with participation from Lockheed Martin Ventures and other investors. Founders Justin Fiaschetti and Justin Briggs both have engineering degrees from Boston Univ.

Skyeports (Sacramento, CA) – Constructing a large-scale, lunar glass habitat in a low-gravity environment. Nicknamed LUNGS (Lunar Glass Structure), this approach involves melting lunar glass compounds to create a large spherical shell structure. This idea offers a promising solution for establishing self-sustaining, large-scale habitats on the lunar surface. Founder Martin Bermudez has a degree in chemistry from UC Berkeley.
Relativity Space (Long Beach, CA) – Commercial reuseable rocket launch using 3D printed rockets. Cofounded by Jordan Noone, A USC trained engineer and Tim Ellis, also a USC engineering grad.
Shield AI (San Diego, CA) – AI pilot systems for aircraft, including drones. Cofounder Ryan Tseng has an EE degree from U Florida and an MBA from MIT; Brandon Tseng a B.S. in Mechanical Engineering from the U.S. Naval Academy and his MBA from Harvard Business School.
Spacium (Long Beach, CA) – In space refueling and repair stations. Cofounder Ashi Dissanayake is a grad of Syracuse Univ and Reza Fetanat is a grad of the Univ Ottawa.
AstroForge (Seal Beach, CA) – Mining asteroids. Founded by Matthew Gialich who has an MS from Cal Poly and Jose acain has an MS from Santa Clara Univ.
Phase Four (Hawthorne, CA) – Developer of satellite propulsion systems designed to economically advance space missions. The company’s systems are small, low in mass, with a thrust-to-power ratio, and with a patented magnetic nozzle to direct thrust, enabling clients to reduce costs and maneuver their satellites into elliptical, geostationary, and polar orbits. Founder Simon Halpern has BS and MS aerospace engineering degrees from U. Michigan and J Sheenan a degree in physics.
Muon Space (Mountain View, CA) – They design, build, and operate low Earth orbit (LEO) satellite constellations optimized for Earth intelligence missions. Founder Jonny Dyer has BS and MS degrees from Stanford, Dr. Paul Day a PhD from Stanford, Dr. Dan McCleese a PhD in phsycis from Oxford, Dr. Reuben Rohrschneider a PhD from Georgia Tech, and Dr. Pascal Strong a PhD from Stanford.
LTA Research (Mountain View, CA) – Founded in 2015 by CEO Dr. Alan Weston, Ph.D., believes that through a combination of new materials, better construction techniques, and other technological advancements, airships are poised to play an important role in society. Dr. Weston, born in Toronto, Canada, completed his undergraduate studies in Engineering Science at Oxford University, and his Master’s Degree and Doctoral Degrees in Aerospace Engineering at Virginia Tech.
Airhart Aeronautics (Long Beach, CA) – new personal aircraft, the Airhart Sling, is designed to be user-friendly, safe, and as easy to learn as possible. Using a single stick, pilots simply point in the direction they want to go and the plane follows, even during takeoffs and landings. The Sling’s computer system translates these controls into commands to the engine and flight systems. The first test flight is planned for 2025, with orders shipping to customers in 2026. Founder Nikita Ermoshkin has a degree in Electrical and Computer Engineering from Cornell University and Brendan Quinn graduated with a degree in Computer Science and a vector in AI from Cornell University. Brendan led Cornell University Unmanned Air Systems
Overair (Santa Ana, CA) – Building 6 passanger eVTOL.
Wisk Aero (Mountain View, CA) – Autonomous eVTOLs.
Jump Aero (Petaluma, CA) – eVTOL for rapid emergency response.
Aska Fly (Mountain View, CA) – eVTOLs that can also drive on raods.
Millennium Space Systems (El Segundo, CA) – They build satellites. Founder Stan Dubyn has a BS and MS in Aerospace Engineering from the University of Southern California.
Viridian Space Corp. (El Segundo, CA) – Viridian’s Air-Scooping Electric Thruster technology – ASET – enables a refuelable satellite platform that flies at Very Low Earth Orbit (VLEO) and transforms the upper atmosphere into an infinite source of propellant. Founder Dr Rostislav Spektor and Dr. Matthew Feldman both have a PhD from Princeton.
LeoLabs (Menlo Park, CA) – Full stack mission management for space launches. Founded by Dr. Daniel Creperly, Ph,D. a grad of UC Berkeley EECS, and John Buonocore who has a BS in Electrical Engineering from San Francisco State University.
ZeroAvia (Hollister, CA) – Hydrogen-electric engines for zero-emission flight. Founder Dr. Val Miftakhov has a PhD in Physics from Princeton University
Beacon AI (San Carlos, CA) – In-flight AI assistance for pilots of aircraft. Founder Dr. Neal Jean has a PhD in computer science from Stanford.
Longshot Space (Oakland, CA) – Building the world’s largest gas gun to provide affordable, responsive hypersonic testing, with the goal of launching cargo into space. Cofounder Mike Grace is a grad of San Jose State University and Nathan Saichk is a grad of California Polytechnic State University-San Luis Obispo.
Impulse Space (Redonod Beach, CA) – In space propulsion systems. Founded by Tom Mueller, BS and MS in engineering from Loyola Marymount University in Los Angeles, and cofounder and lead engineer at SpaceX.
Rain Aero (Alameda, CA) – Autonomous helicoptors for fire detection and fighting.
Vestigo Aerospace (La Canada-Flintridge, CA) – Deorbit technology for satellites. Founded by Dr. David Spencer, Ph.D., received his B.S. and M.S. degrees in Aeronautics and Astronautics from Purdue University, and his Ph.D. from Georgia Tech.
Natilus (San Diego, CA) – San Diego has a long tradition of building the most advanced aircraft in the USA, such as the B-58, the world’s first supersonic bomber and the first to reach Mach 2. In the tradition of San Diego’s aircraft innovation, Natilus is a San Diego–based aerospace company developing next-generation blended-wing-body cargo aircraft.

JetZero (Long Beach, CA) – Another company designing blended-wing commercial jets. Significant investment has come from a number of sources, including Alaska Airlines.
JetZero’s 1/8 size demonstrator plane has been granted an FAA Airworthiness certificate and test flights have begun.
Joby Aviation (Marina, CA) – eVTOLs for commerical passenger use. They are well on their way to receiving certification to carry passengers in the USA, and just received more investment from Toyota. Their founder, CEO, JoeBen Bevirt has a BS from UC Davis and an MS from Stanford.
Capella Space (San Francisco, CA) – Advanced radar systems for space. Founded by Frank Backes who has an electrical engineering degree from UC San Diego.
Apex Space (Los Angeles, CA) – Standard satellite bus models, satellite platforms, from 100 to 500kg, configurable to mission needs. Ian Cinnamon is the founder, a graduate of MIT.
Archer Aviation (San Jose, CA) – eVTOLs for commerical passenger use.
CesiumAstro (El Segundo, CA) – Phased array communication systems for space and air vehicles. Founded by Dr. Mike Griffen, Ph.D., a graduate of USC and Univ Maryland, and Dr. Lisa Porter, Ph.D., a graduate of MIT and Stanford.
Wisk Aero (Mountain View, CA) – autonomous eVTOLs for commerical passenger use.
Elroy Air (South San Francisco, CA) – autonomous eVTOL for deliveries of 300lb paylods.

Innoflight (San Diego, CA) – Avionics for spaceships and satellites.
Alef Aeronautics (San Mateo, CA) – Flying electric cars, seriously. And they have thousands of pre-orders.
Momentus Space (San Jose, CA) – “Space infrastructure” services, including space transportation, on-orbit refueling, and on-orbit services of satellites. Founded by Mikhail Kokorich, physics and business degrees from Russia.
Muon Space (Mountain View, CA) – Low Earth orbit satellite constellations. Cofounded by Jonny Dyer, BS and MS dregrees from Stanford, Dr. Paul Day, PhD, Stanford, and Dr. Dan McCleese, PhD from Oxford.
NovaWurks (Los Alamitos, CA) – Bespoke space engineering. Founded by Talbot Jaeger, BS from UC Irvine.
Northwood Space (El Segundo, CA) – Building a global network to send data for satellites, built using phased array technology that we have now successfully validated, both in the lab and in the field. A phased array is a group of sensors located at distinct spatial locations in which the relative phases of the sensor signals are varied in such a way that the effective propagation pattern of the array is reinforced in a desired direction and suppressed in undesired directions. Cofounded by actress/singer Bridgit Mendler who has a MS from MIT.
Turion Space (Irvine, CA) – Microsats for sensing. Cofounded by Ryan Westerdahl, BS and MS degrees from Univ. Washington; Tyler Pierce, graduate of UCSD; Patryk Wiatr, BS from Central Washington U.
Tyvak (Irvine, CA) – Manufacturer of nano and micro satellites. Cofounded by Dr. Jordi Puig-Suari, PhD, professor at Cal Poly.
Northwood Space (El Segundo, CA) – Earth to space communications.
ABL Space Systems (El Segundo, CA) – Small, Mobile Launch Systems and Rockets
AEM (San Diego, CA) – Electronic parts for space ships, satellites
AeroFoam (Lake Elsinore, CA) – Materials for aerospace cabins.
Aevex Aerospace (Solana Beach, CA) – Space Data Systems and Analysis
K2 Space (Torrance, CA) -Large satellites. Founded by Karan Kunjur and Neel Kunjur, both are graduates of Northwestern University
Xona Space Systems (Burlingame, CA) – Positioning, navigation and timing (PNT) satellites. Cofounder Dr. Tyler Reid has a Ph.D. in Aeronautics and Astronautics from Stanford University, and Brian Manning has a MSc in Aeronautics and Astronautics from Stanford University.
AnySignal (El Segundo, CA) – Aerospace communications. Cofounder John Malsbury is a grad of embryRiddle Univ in Long Beach and Dr. Ricardo Medina has a PhD from Stanford.
Astra (Alameda, CA) – Small satellite propulsion systems. Cofounder Dr. Adam London has a PhD from MIT.
Astrolabs Venturi (Hawthorne, CA) – Space land rovers. Founder Jaret Matthews has a BS degree from Purdue and an MS from Stanford.
Capella Space (San Francisco, CA) – Imaging anytime, in any weather, with advanced Synthetic Aperture Radar (SAR) satellites. Founded by Payam Banazdeh who has an MS from Stanford.
Varda Space (El Segundo, CA) – Microgravity-enabled life sciences company that processes materials in orbit and returns them to Earth. Founded by Will Bruey, BS and MS degrees from Cornell, and Delian Asparouhov a grad of MIT.
Virgin Galactic (Tustin, CA) – Develops commercial spacecraft. Cofounded by Burt Rutan, a grad of Cal Poly.
Vast Space (Long Beach, CA) – They build space stations. Founded by Jed McCaleb, who attanded UC Berkeley but did not finish.
Stratolaunch (Mojave, CA) – Hypersonic aircraft and launch vehicles. CEO is Dr. Zachary Krevor, Ph.D, degrees from UCLA and Georgia Tech.
Stellar Exploration (San Luis Obispo, CA) – Nano satellite and propulsion systesms. Founder Dr. Tomas Svitek, Ph.D. is a grad of Caltech.
Space Micro (San Diego, CA) – Space Communications. Digital Systems. Electro-Optics.
InterOrbital Systems (Mojave, CA) – Rocket, satellite, and spacecraft manufacturing company and launch-service provider
AstroBotic (Mojave, CA) – Reusable space vehicles with the most rocket-powered landings in the industry
Wing Delivery (Mountain View, CA) – Drone deliveries, such as blood in London.
Xona Space Systems (Burlingame, CA) – Modern technologies by providing precise and protected navigation services anywhere on Earth. Xona’s PULSAR service provided through a constellation of cutting edge small satellites will form the foundation to support autonomy at scale. Cofounded by 6 people, all with PhDs or Masters degrees.
Orbital Operations (Huntington Beach, CA) – In-space cryo management tech to unlock high thrust, high performance engines beyond earth’s atmosphere. Their vehicle is designed to operate permanently in space to transport customers from LEO to MEO, GEO, and the Moon. Cofounders Ross Doherty has an engineering degree from Case Western and Ben Schleuniger an engineering degree from UC Riverside.
SpinLaunch (Long Beach, CA) – Instead of relying on traditional rocket propulsion, SpinLaunch’s approach uses kinetic energy to fling specially designed microsatellites—flattened like oversized pancakes—into the upper atmosphere. These 7.5-foot-wide satellites weigh around 150 pounds each and are built to endure staggering acceleration forces of up to 10,000 Gs. This makes them far lighter and more compact than most competitors’ designs.
Zipline (South San Francisco, CA) – Drone delivery systems. Founded by Keller Rinaudo Cliffton, a Harvard grad, Dr. Keenan Wyrobek, PhD who began his robotics journey at Stanford University, where he earned a Master’s in Mechanical Engineering and a Ph.D. in Computer Science. During his academic career, he co-founded the Personal Robotics Program at the Stanford AI Lab., Ryan Oksenhorn, a grad of Carneige Mellon, and Will Hetzler, a grad of Harvard.
Impulse Space (Redondo Beach, CA) – Vehicles that move payloads across and between orbits — rapidly, reliably, and affordably. Founded in 2021 by propulsion legend Tom Mueller, cofounder of SpaceX and developer of its technology, who has BS and MS degrees in aeronautical engineering from Idaho University and Loyola MaryMount Univ.
Batteries
Chemix AI (Sunnyvale, CA) – A spinout of UC Berkeley’s SkyDeck, they do AI battery design. BTW, UC Berkeley is the #1 school for producing tech founders (Stanford is #2), and SkyDeck is one reason. Chemix was founded in 2021 and uses generative AI to design next-generation batteries. The company’s mission is to create sustainable and high-performing battery chemistries. Chemix’s AI-based platform, MIX, is said to be 10 times faster than conventional battery development processes, and has led to innovations such as removing cobalt from lithium-ion batteries. Cofunded by Dr. Kaixiang Lin, PhD (Stanford) and Dr. Jason Koeller, PhD (Berkeley).
Sila Nano (Alameda, CA) – Sila is the first to deliver a market-proven nano-composite silicon anode that powers breakthrough energy density, without compromising cycle life or safety. Cofounder Gene Berdichevsky has BS and MS degress from Stanford, Gleb Yushin, graduate degree from Georgia Inst. Tech.
Amprius (Freemont, CA) – Silcone anode batteries giving a high yield and 90% charge in 15 minutes. Prof. Dr. Yi Cui, Ph.D. founded Amprius iand is a professor and the Director of the Stanford Sustainability Accelerator at Stanford University.
Sakuu ( San Jose, CA) – 3D printed batteries. Sakuu’s dry-process manufacturing innovations include the first fully functional printed lithium-metal battery, the first printed and patterned lithium-metal anode, and the first printed lithium-metal battery in a custom form factor. The Kavian battery manufacturing platform is advancing battery manufacturing and is being licensed to other companies, large battery manufacturers. Founder Robert Bagheri is a grad of Cleveland Institute of Electronics.
QuantunScape (San Jose, CA) – Solid state lithium ion batteries
Inlyte Energy (San Leandro, CA) – Sodium metal halide grid batteries designed to provide cost-effective energy storage. The company focuses on using abundant, low-cost materials like iron and sodium to create safe, long-lasting batteries capable of withstanding extreme conditions. Founded by Dr. Antonio Baclig, Ph.D. a grad of Stanford.
Antora Energy (Sunnyvale, CA) – Carbon heat blocks for energy storage. Able to transfer heat, at 90% effiency, or electricty at about 40% effiiency, for industrial use. Backed by a number of groups, including ARPA-E, and already delpoyed in commercial applications.
Rondo Energy (Oakland, CA) – Carbon heat blocks for energy storage, called heat batteries. They already have commercial units in operation throughout the USA and Europe.
South 8 Technologies (San Diego, CA) – A spinout of UCSD, the company pioneered LiGas®, a liquefied gas electrolyte that dramatically improves the energy performance of lithium-ion batteries for next-generation EVs and other industrial applications.
And Battery Aero (Palo Alto, CA) – Batteries for aerospace. Founded by Dr. Shawshank Sripad, PhD, educated at Carnegie Mellon.
Life Sciences, Medical and Health
Foundery Innovations (San Francisco, CA) – Foundery’s co-development venture studio model aligns first-in-class drug development opportunities with financing and experimental execution to catalyze university discoveries. Founded in 2021 by science-first industry leaders Prof. Dr. Max Krummel, Ph.D., Dr. Michel Streuli, Ph.D., and Dr. Venkataraman “Sriram”, Ph.D., Foundery seeks to validate early-stage immunotherapy programs in collaboration with university researchers and their institutions.
BIOTICSai (Oakland, CA) – Fetal ultrasound screening using AI.
Addition Therapeutics (Berkeley, CA) – Used to treat chronic, rare genetic diseases, their core technology is its all-RNA, nonviral, lipid nanoparticle-based PRINT (Precise RNA Mediated Insertion of Transgenes) platform based on the retrotransposase work Of Prof. Dr. Kathleen Collins, Ph.D. at UC Berkeley.
Chai Discovery (San Francisco, CA) – Develops artificial intelligence models that predict and reprogram biochemical molecule interactions. Founded by Dr. Joshua Meir, Ph.D. who has a PhD from Harvard, and Jack Dent who has an MS in computer science from Harvard,
ResVita Bio (Berkeley, CA) – Synthetic biology using skin derived microbes to produce skin therapeutics. Founded br Dr. Amin Zarger, PhD, a postdoc at UC Berkeley in the lab of his cofounder, Prof. Dr. Jay Keasling, PhD of UC Berkeley.
EditPep (Berkeley, CA) – CRISPR therapies enabled by a simple, potent delivery platform. The biotechnology company creates a class of peptides capable of delivering CRISPR enzymes into clinically-relevant and previously untransfectable cell types, enabling clients to get CRISPR therapies. Cofounded by Prof Dr. Ross Wilson, Ph.D., professor of Molecular and Cell Biology at UC Berkeley, and Dr. Dana Foss, Ph.D. a fellow at UC Berkeley.
HOPO Therapeutics (Berkeley, CA) – Developing pharmaceuticals designed to treat exposure to heavy metals, beginning with our flagship drug candidate HOPO-101, now in clinical development. Cofounded by Dr. Julian Reese, Ph.D. who received a B.A. in Chemistry from Goucher College, earned his M.S. and Ph.D. in Chemistry from the University of Washington, and has held research fellowships at the Max Planck Institute for Chemical Energy Conversion, the University of California, Berkeley, and Lawrence Berkeley National Laboratory. Julian is currently an Activate Fellow at Berkeley Lab’s Cyclotron Road, and Prof. Dr. Rebecca Abergel, Ph.D. who received her B.Sc. in Chemistry from the Ecole Normale Supérieure in Paris, France, her Ph.D. in Chemistry from the University of California, Berkeley, and Hannah Weber who earned her B.S. in International Health at Georgetown University, an MBA at the UC Berkeley Haas School of Business, and MPH from the UC Berkeley School of Public Health.
Profluent Bio (Berkeley, CA) – Combines machine learning and biology to unlock solutions to complex challenges in protein design. Founded by Dr. Ali Madani, Ph.D., a Berkeley grad; Dr. Alexander Meeske, Ph.D, a grad of Univ. Washington.
Regenerative Patch Technologies (Portola Valley, CA) – Stem cell-based technologies for retinal diseases, such as dry macular degeneration. Founded by Prof. Dr. Dennis Clegg, Ph.D., professor at UC Santa Barbara who attained his doctorate at UC Berkeley, and developed the stem cell technology at UCSB.
Foodsmart (San Francisco, CA) – Telenutrition and foodcare solution, backed by a robust network of registered dietitians. Their platform is designed to foster healthier food choices, drive lasting behavior change, and deliver long-term health outcomes. Through their highly personalized, digital platform, they guide our 2.2 million members—including those in employer-sponsored health plans, regional and national Medicaid managed care organizations, Medicare Advantage plans, and commercial insurers—on a tailored journey to eating well while saving time and money. Founded by Dr. Jason Langheier MD, MPH, who has an MD from Duke and an MPH from Harvard.
Poseida Therapeutics (San Diego, CA) – Immune cell therapies against several types of blood cancers. Roche has announced plans to aquire Poseida. Cofounded by Dr. Kristin Yarema, Ph.D., a doctor of Chemical Engineering from the University of California, Berkeley.
CircuCare (San Diego, CA) – CircuCare is advancing patient care with a state-of-the-art wearable ultrasound device that provides automated, continuous medical imaging. Founded by Dr. Sai Zhou, PhD, who earned his doctorate in the Materials Science and Engineering program at UC San Diego.
Persperion (San Diego, CA) – Persperion’s test strips can measure biomarkers in perspiration, including metabolites, electrolytes, hormones, drugs and beyond, from a touch of your fingertip. Founded by Dr. Lu Yin, PhD, who earned his Ph.D. and M.S. in Nanoengineering from UC San Diego.
Treeline Biosciences (San Diego, CA) – They’re focused on targeting proteins involved in cancer. Treeline Biosciences brought in the second largest life sciences fundraise in 2024, tapping 43 unnamed investors for a nearly $422 million financing round. Founded by two physicians, it’s unclear at this time what they’re doing beyond targeting proteins.
Mirador Therapeutics (San Diego, CA) – Mirador secured a massive $400 million series A in the first quarter of 2024. They are developing precision medicine for immune-mediated inflammatory and fibrotic diseases.
Navega Therapeutics (San Diego, CA) – Epigenome regulation for pain reduction. Founded by Dr. Ana Moreno, Ph.D., a grad of UCSD.
Candid Therapeutics (San Diego, CA) – They develop T-cell engagers, which are bispecific antibodies that bind to T cells and a molecule on a target cell so the T cells attack the target. Raised $370 million in 2024.
Arnatar Therapeutics (San Diego, CA) – Based on decades of experience in antisense technology and RNA biology, Arnatar enhances its siRNA molecules with precise chemical changes to improve potency, specificity, stability and safety. Founders Dr. Xuehai Liang, Ph.D. and Dr. Xuehai Liang, Ph.D. have years of successful experience in developing RNA therapeutics. In August 2025, Arnatar received $52 million in series A funding and a pair of designations from the FDA it hopes will smooth the clinical and regulatory path forward for one of its RNA-based therapeutics.
Circular Genomics (San Diego, CA) – They recently moved from New Mexico to San Diego. Circular RNA for improving the diagnosis and treatment of psychiatric and neurological disorders. Founded by Dr. Alex Hafez, PhD, MBA, a grad of the Univ. New Mexico.
Nanovision (San Diego, CA) – Artificial retinal implants composed of nanoelectronics. A private-public partnership with UCSD.

Maze Therapeutics (South San Francisco, CA) – Maze Therapeutics thinks differently about genetics by developing medicines designed to imitate the effects of rare, naturally occurring, protective genetic variants and help halt the progression and potentially reverse the effects of kidney disease. Founder Dr. Jason Coloma, PhD, has a Ph. D. and M.P.H. from the University of California, Berkeley, an MBA from the Tuck School of Business at Dartmouth and a B.S. in biology from the University of San Francisco.
ArsenalBio (South San Francisco, CA) – Programmable cell therapy company focused on engineering advanced CAR T-cell therapies for solid tumors. Founded by Prof. Dr. E. John Wherry, Ph.D. who is Distinguished Professor, Chair of the Department of Systems Pharmacology and Translational Therapeutics in the Perelman School of Medicine and Director of the UPenn Institute for Immunology and Ken Drazan MD.
Vaxart (S. San Francisco, CA) – Their vaccines act differently, by attacking invading pathogens in the places where they first enter the body: the mucosal areas, including the mouth, the nose and the gut instaed of typical IM administration. They are designed to trigger strong IgA and T-cell responses to repel and overwhelm the invading viral invaders. Founded by Dr. Sean Tucker, PhD, who has a BSc. in chemical engineering from the University of Washington, an MSc. in chemical engineering from the University of California, Berkeley, and a Ph.D. in immunology from the University of Washington.
CRISPR QC (San Diego, CA) – Real-time QC of CRISPR gene editing. Cofounded by Prof. Dr. Kiana Aran, PhD, a grad of UC Berkeley and professor at UCSD.
Alterome (San Diego, CA) – Targeted oncology therapies using a drug discovery platform based on a powerful computational chemistry engine that applies state-of-the-art, physics-based molecular simulations to proprietary co-crystal structure data. Founded by Dr. Eric Murphy, Ph.D. and Dr. Ryan Corcoran, MD, PhD from Mass General Hospital, and Aaron David who holds an M.S.in biotechnology from Columbia University and a B.A. in business administration from Emory University.
Neurona Therapeutics (South San Francisco, CA) – Stem cell therapeutics for nervous system diseases. They use a technology developed by cofounder, Prof. Dr. Arturo Alvarez-Buylla, PhD. of UC San Francisco.
Merryfield Therapeutics (Menlo Park, CA) – Scientists at Stanford and Berkeley discovered a peptide molecule, BRP, that acts through a separate but similar metabolic pathway to commercial GLP-1 agonists and activates different neurons in the brain — offering a more targeted approach to body weight reduction. Prof. Dr. Katrin J. Svensson, PhD of Stanford led the research and founded the company.
Boundless Bio (San Diego, CA) – Cofounder Dr. Chris Hassig, PhD co-discovered that ecDNA non-randomly transcribe and render tumors resistant by facilitating massive oncogene transcription- Dr. Hassig has found a way to target this process. Dr. Hassig earned a B.A. in Chemistry and Biochemistry from the University of California, San Diego, and a Ph.D. in Molecular and Cellular Biology from Harvard University. He completed a Postdoctoral Fellowship at the University of California, Berkeley. he has an academic appointment at Scripps Research Institute in San Diego.
VisiCell (San Diego, CA) – Labeling cells that have been administered to patients. Often, cell therapies have no idea where the cells are in the body or if the cells are alive. Founded and led by Mya Thu, M.Sc., MBA, degrees from USC and UCSD.
Regenerative Patch Technologies (Portola Valley, CA) – Stem Cell-based technology for dry macular degeneration. Technology developed by Prof. Dr. Dennis Clegg, PhD, UC Santa Barbara, who received a PhD in biochemistry at UC Berkeley.
CytomX (South San Francisco, CA) – Drug designs that selectively activate in the tumor microenvironment while reducing drug activity in healthy tissue and in circulation. Cofounded by Professor Dr. Patrick Daugherty, PhD of UC Santa Barbara.
Strava (San Francisco, CA) – Fitness and health apps.
Conception (Berkeley, CA) – Turning stem cells into human eggs to facilitate conception. Cofounded by Dr. Pablo Hurtado, Ph.D., alum of Univ Edinburgh.
Science Corporation (Alameda, CA) – Neural engineering. They currently have a electronic prothesis for retinal degeneration in clinical trials. Cofounded by Max Hodak, a cofounder of Neuralink and a grad of Duke University engineering, and Prof. Dr. Tyson Kim, MD, PhD who has an MD from UCSF and a PhD from UC Berkeley.
Valora Therapeutics (San Diego, CA) – Targeting specific sugar molecules on cells, AbLecs modulate glyco-immune checkpoints—a key control point in the body’s immune response. This innovative approach holds significant potential for developing first-in-class and best-in-class therapeutics in oncology, autoimmune diseases, and other therapeutic areas. Founded by Prof. Dr. Carolyn Bertozzi, PhD, a grad of UC Berkeley where she was also a professor, and now at Stanford.
CatenaBio (San Diego, CA) – Making conjugated antibodies for enhanced therapeutic actions. UC Berkeley business and chemistry alumni Geo Guillen and Dr.Marco Lobba, Ph.D., launched Catena Biosciences with Berkeley chemistry professor Prof. Dr. Matthew Francis, Ph.D.
Olfera (Mountain View, CA) – Nasal delivery of drugs through the olfactory system’s connection to the brain. Led by cofounder Dr. Pernian Lak, Ph.D., a UCSF alum.
Air Surgical (San Diego, CA) – Precision robotic surgical devices. Co-founded by Dr. Dimitri Schreiber, Ph.D., who earned both bachelor’s and master’s degrees in electrical engineering at UC San Diego and completed his Ph.D. in the Advanced Robotics and Controls Lab at UCSD.
Eko Health (Emeryville, CA) – Combining ECG-enabled digital stethoscope devices with monitoring and analysis algorithms. These tools are designed to help providers detect heart rhythm abnormalities and a range of other heart diseases, and to support the company’s recently launched telehealth platform.
Nanome (San Diego, CA) – Multi-user AI platform for spatial analysis in drug discovery. Founded by four UCSD graduates, including one who majored in nanoscience.
Palage Pharmaceuticals (Los Angeles, CA) – Using a small molecule discovered at UCLA to drive stem cell activity that results in hair growth. Founded by Prof. Dr. Heather Christofk, PhD, Prof. Dr. Michael Jung, PhD, and Prof. Dr. William Lowry, PhD, all at UCLA in the departments of chemistry and molecular biology.
AsparaGlue (Berkeley, CA) – AsparaGlue develops a superglue-like medical adhesive that can be uniquely applied for external wound closure and internal tissue adhesion. Co-Founded by Dr. Phillip Messersmith, Ph.D., Professor of Bioengineering and Materials Science and Engineering at UC Berkeley, and currently chair Dept. of Bioengineering.
NeoGenesis (San Diego, CA) – They use a new methodology to create healthcare products for skin, immune system, and nervous system, developed by Prof. Dr. Greg Maguire, Ph.D. a former bioengineer ar UC Berkeley and professor at UCSD. A systems therapeutic approach to renormalize physiology is used. Radiation dermatitis in cancer patients, immune system vaccination, and neurodegenerative diseases have benefited from their approach. Adult stem cell released molecules are part of their core technology.
Seer Proteomics (Redwood City, CA) – Deep, unbiased proteomic analysis. Founded by Dr. Omid Farokhzad, MD, MA, Dr. Philip Ma, PhD, a grad of MIT, and Dr. Robert Langer, PhD, a professor at MIT.
RayThera (San Diego, CA) – An early-stage biotech focused on immunology raised $110M in a series A. Cofounded by Dr. Qing Dong, Ph.D. and Dr. Gene Hung, M.D. who did a postdoc at USC.
Biolinq (San Diego, CA) – A startup that develops wearable biosensors raised a $100M Series C. Founders Rich Yang received his B.S. degree in Biological Sciences from the University of California, Irvine, and Dr. Jared Tangney, PhD received his Ph.D. in Bioengineering from the University of California, San Diego, and was a Business Technology Fellow at the University of California, San Diego Jacobs School of Engineering.
Element Biosciences (San Diego, CA) – Integrated platform for high-quality affordable, sequencing and in situ multiomics. Founded by Dr. Molly He who has a PhD from the University of California, Los Angeles in protein biophysics, and Dr Michael Previte, PhD who has PhD from Boston College in physical chemistry and received his postdoctoral training from MIT.
C. Light Technologies (Berkeley, CA) – Co-founded by UC Berkeley alumni Dr. Christy Sheehy, Ph.D. and Dr. Joe Xing, PhD. Through her dissertation as a Berkeley Ph.D. student in vision science, Sheehy created technology that can assess a patient’s neurological health through a 10-second high resolution retinal screening.
Xaira (San Francisco, CA) – AI for understanding proteins, the key set of molecules underlying diseases. What I like about this company, is they don’t focus on genetics, which has little to do with disease, but focus on proteins. The company was co-founded by Prof. Dr. David Baker, Ph.D., professor of biochemistry and director of the Institute for Protein Design at the University of Washington School of Medicine. Dr. Baker received his Ph.D. from UC Berkeley.
iCardio.ai (Los Angeles, CA) – AI ultrasound for cardiology.
Science (Oakland, CA) – Technologies required for modern neural engineering, enabling devices aimed at restoring vision, communication and cognition. Acquired electronic retinal implant for low vision, such as macular degeneration.
Cellanome (Foster City, CA) – Multiomics platform enables iterative live cell phenotypic and functional assays, at scale, linking each cell’s response to specific perturbations. This allows for a more granular view of the heterogeneity of cellular responses, highlighting individual cellular responses over bulk measurements, and facilitating combinatorial assays without needing to switch platforms or consume additional sample. If you’ve heard of San Diego’s genome sequencing giant, Illumina, this could be the next generation omics platform that surpasses Illumina. Founded by Dr. Mostafa Ronaghi, PhD, formerly of Illumina and a scientists at Stanford.
Ultima Genomics (Freemont, CA) – Low-cost sequencing using a new sequencing architecture that scales far beyond conventional technologies. Founded by Dr. Gilad Almogy, PhD, who earned a PhD in Applied Physics from the California Institute of Technology.
Element Biosciences (San Diego, CA) – In 2024 Element released a machine that generates multiomic data via a relatively seamless process — which the company says saves time, money and cuts down on biological noise from stitching together so many data sources. Founder Dr. Molly He, PhD holds a PhD from the University of California, Los Angeles in protein biophysics, Dr. Michael Previte, PhD holds his PhD from Boston College in physical chemistry and received his postdoctoral training from MIT, and Dr. Matthew Kellinger earned his PhD at the University of Texas in Austin where he focused on mechanisms of Reverse Transcriptase and HIV drug resistance. He continued his research at UC San Diego where he studied RNA transcription fidelity.
Environmental
Earth Species Project (Berkeley, CA) – They use artificial intelligence to figure out how animals communicate. Imagine if we could understand what the animals are communicating to one another. Cofounded by Katie Zacarian, an alum of Harvard; Aza Raskin, an alum of U. Chicago and a PhD student at Caltech; and Britt Selvetelle, alum of U. Kentucky.
Plastic Beach (San Diego, CA) – Recycling of soft, “non-recyclable” plastics.
Deep Isolation Nuclear Technology (Berkeley, CA) – Disposing of nuclear waste deep underground. Founded by Prof. Dr. Richard Muller, PhD, professor of physics at UC Berkeley.
Charm Industrial (San Francisco, CA) – Charm uses plants to capture CO₂ from the atmosphere. We convert biomass into a stable, carbon-rich liquid and then pump it deep underground. This removes CO₂ permanently from the atmosphere, out of reach of wildfires, soil erosion and land use change. Currently partners with Google.
Vibrant Planet (Truckee, CA) – Founded by Allison Wolff, the company develops cloud-based software for utilities, insurers, and land managers like the U.S. Forest Service to model and respond to wildfire risk. To ensure the company keeps its eye on the mission, it has registered as a public benefit corporation, which requires companies to report on impact in addition to the usual financial information.
Mill (San Bruno, CA) – In home food recycling producing compost. Founded by Matt Rogers, Carnegie Mellon University grad with BS & MS (Electrical & Computer Engineering)
Stax Engineering (Long Beach, CA) – STAX Engineering is a privately held company that specializes in environmental services, including air pollution control, shore power, and greenhouse gas reduction. Their emission capture and control system is used in the Ports of Los Angeles, Long Beach, and Oakland, and they are expanding to other California ports
CarbonBlade (San Diego, CA) – Zero Emission Direct Air Capture (DAC) system sequestering CO2 at the source. Cofounded by Hunaid Nulwala, Ph.D.
Planet Labs (San Francisco, CA) – High-resolution daily Earth satellite data, archive, and analytics give customers an unprecedented view, allowing them to cast further and wider in time, space, and frequency. So they can make decisions and take confident action. They manufacture and launch small satellites to support their dat gathering platform. Cofounded by Dr. William Marshall who has a PhD from Oxford.
Wild Genomics (San Diego, CA) – Using genomic data in the field to analyze pest invasion in crops to optimize what to use for extermination. For example, by pinpointing where the pest problem is on the farm, farmers don’t have to spray the whole field. Founded by Drs. Eirik Torheim, PhD and Bilgenur Baloglu, PhD.
Heirloom Carbon Technologies (Brisbane, CA) – Established America’s first commercial Direct Air Capture (DAC) facility in the Central Valley of California. They use limestone, one of the world’s most abundant and inexpensive minerals, to capture CO2 directly from the atmosphere, and then permanently and safely store it underground. Cofounder Dr. Noah McQueen, Ph.D. attained his PhD at U. Penn.
NexusFlow (Palo Alto, CA) -Builds LLM agents using technology originally developed at Berkeley by Prof. Dr. Jiantao Jiao that specializes in cybersecurity.
Kodama AI (Sonora, CA) – AI technology for forest management, including wildfire mitigation.
Malin Space Science Systems (San Diego, CA) – Imaging systems for space from Dr. Mike Malin, Ph.D., a Berkeley and CalTech grad.
Natron Ebergy (Santa Clara, CA) – Sodium batteries for industrial applications like AI, data centers, peak shaving, and power quality management
Databricks (San Francisco) – Built by the guys (faculty and their students) from UC Berkeley who created Apache Spark, they went commercial with Databricks. Organizing diverse data sets and making them actionable, including with the use of AI, is what this billion dollar unicorn does.
Logistics
Flexport (San Francisco, CA) – End-to-end supply chain, all in one platform. Ryan Petersen, the founder and CEO of Flexport, is a UC Berkeley grad.
Platform Sciences (San Diego, CA) – From the back office to the driver’s seat, they provide integrated solutions at every level to help fleets future-proof their operations.
Zeem (El Segundo, CA) – Zeem is a turnkey solution for changing commercial fleets into an all-electric powerhouse
Automotive and Transportation
Aptera Motors (San Diego, CA) – Electron efficient solar cars. San Diego is the greenest city in the USA, and this car will make it greener. Chris Antony is a grad of UNC and Steve Fambro has a BSEE degree from the University of Utah

Rivian – (Irvine, CA) – Another company that moved its HQ to California, they make electric trucks.. Dr. Robert Joseph “RJ” Scaringe, Ph.D., attained his PhD at MIT, is the founder and CEO.
Parallel Systems (Los Angeles, CA) – Electric powered, autonomous trains. Parallel has raised $100 million and received approval from the Federal Railroad Administration to operate on shared railways. The train cars are about 44 feet long and sit low to the ground, ready to be top-loaded with a cargo container. Slightly shorter than a standard 50-foot freight car, the Parallel cars weigh roughly 40,000 pounds unloaded. Founder Matt Soule holds advanced degrees in Electrical Engineering from the University of Southern California and Northwestern University; founder John Howard has M.S. from Stanford University and B.S. Engineering Physics at Loyola Marymount University; founder Ben Stabler Stanford University School of EngineeringMaster of Science (M.S.) (Electrical Engineering)2012 – 2015,Stanford UniversityBachelor of Science (B.S.) (Computer Science); and founder Brian Ignaut is a grad of U. Michigan.
Also Inc (Irvine, CA) -Micromobility spinout from Rivian with multiple investors.
Telo Trucks (San Carlos, CA) – Electric truck has the footprint of a Mini Cooper SE, but possibly the same utility that one would expect from a larger truck. The $50,000 electric truck is also claimed to be the world’s most efficient. Founder Jason Marks has a BS in engineering from Columbia Univ, Forrest North is a grad of Stanford, and Yves Behar is a grad of California Art Center College of Design.
Lucid Motors (Newark, CA) – Award winning EV that, along with the Porsche EV, is the most advanced EV auto on the planet. Cofounded by Dr. Bernard Tse, PhD, grad of U. Illinois, and Sam Weng a grad of UC Berkeley.
Seasats (San Diego, CA) – Autonomous surface vehicles. Founder Mike Flanigan has a BS in engineering from Notre Dame and Dylan Rodriguez has an engineerinf degree from Worcester Polytechnic Institute.
Telo Trucks (San Carlos, CA) – Efficient EV pickup for urban living and weekend adventuring. Cofounder Jason Marks has an engineering degree from Columbia University, Forrest North is a grad of Stanford, and Yeves Behar is a grad of the Art Center College of Design in Pasadena, California.
Mullen Automotive (Brea, CA) – EVs, cars and commercial trucks.
Aventon Bikes (Brea, CA) – Smart Ebikes.
Zum (Oakland, CA) – They provide an end-to-end, future-ready transportation solution powered by leading-edge platform technology, and analytics-driven operations, electric fleets to transport kids to and from school, and easy-to-use apps so you know how everything is going. Founded by Ritu Narayan, undergrad in India and graduate school at Stanford.
TuSimple (San Diego, CA) – Autonomous trucking. Founded by a Caltech PhD.
Aurora Innovation (Mountain View, CA) – Self driving commercial trucks. Founder Dr. Chris Urmson has a PhD in robotics from Carnegie Mellon, Dr. Sterling Anderson a PhD from MIT, and Dr Drew Bagnell a PhD from Carnegie Mellon.
Navier (San Francisco, CA) – Electric, hydrofoil boats. Founded by Dr. Sampriti Bhattacharyya, Ph.D. She’s a grad of MIT.
Arc Boats (Torrance, CA) – Electric boats with conventional hull. Both founders, Mitch Lee and Ryan Cook have mechanical engineering degrees from Northwestern University.
Glydways (S. San Francisco, CA) – Glydways is a new on demand, anytime, high capacity mobility system. It uses autonomous, personal Glydcars moving riders safely in dedicated lanes. Founder Mark Seeger received a bachelor of science in mechanical engineering and psychology from the Rensselaer Polytechnic Institute in Troy, New York,
Zoox (Foster City, CA) – Autonomous electric cars. Cofounded by Dr. Jesse Levinson, Ph.D., a grad of Princeton and Stanford.
Zoox on the streets of San Francisco.
Waymo (Mountain View, CA) – Robotaxis. Founded by Prof. Dr. Sebastion Thrun, Ph.D., a professor at Stanford.
Cruise (San Francisco, CA) – Autonomous vehicles. founded by Kyle Vogt, an MIT grad and Dan Kan, a grad of Claremont Colleges near Los Angeles.
Zero Motorcycles (Scotts Valley, CA) – Electric motorcycles. Founded by Neal Saiki, who has an M.S. in engineering from California Polytechnical University.
XOS (Los Angeles, CA) – Electric trucks. Cofounded by Dakota Semler, a grad of Cal State U, and Giordano Sordoni, a grad of George Washingtom U.
Mullen Automaotive (Brea, CA) – EV autos and trucks.
Harbinger Motors (Garden Grove, CA) – EV trucks.
Hayden AI (San Francisco, CA) – AI-based mobile perception platform. Founded by Dr. Chris Carson, Ph.D., and incubated at Berkleley Skydeck.
Phoenix EV (Anaheim, CA) – Electric buses.
Cenntro (Ontario, CA) – HQ in NJ, but just opening production facility in Ontario, CA after closing its operations in Florida, a state that has doub;e-downed on petrochemicals.
Platform Science (San Diego, CA) – Technology for fleet management. Cofounded by Jack Kennedy who has adegree form the US Naval Academy and an MBA from Harvard, and Jake Fields who has a BS from Drexel University.
Pony AI (Freemont, CA) – Self-driving technology company that was founded in Silicon Valley in 2016. The company operates fleets of robotaxis and trucks in the US and China. Cofounders Dr. James Peng has a Ph.D. from Stanford, and Dr. Tiancheng Lou has a Ph.D. and a B.S. from Tsinghua University in Beijing, China.
Nevoya (Santa Monica, CA) – Zero-emissions technology and trucking platform. Cofounder Thomas Atwood has degrees from Boston U and Stanford.
WattEV (San Bernadino, CA) – They build EV charging infrastructure, solar- and battery-powered charging hubs.
Imaging
ThinkSite (Redwood City, CA) – Their VisionSort platform combines traditional fluorescence flow cytometry with high-dimensional morphological profiling and AI to enable label-free cell sorting and unbiased single cell profiling. Cofounder by Dr. Sadao Ota, PhD, who has a Ph.D. in engineering from UC Berkeley and a BS from the University of Tokyo and Issei Sato, PhD, who has a Ph.D. in Information Science and Technology from the University of Tokyo.
Opal Cameras (San Francisco, CA) – Small, inexpensive, high resolution video cameras. Cofounded by Veeraj Chugh, A USC grad, and Stefan Sohlstrom, a Univ Oregon grad.
Orbital Sidekick (San Francisco, CA) – Satellite-based spectral imaging of Earth. Founder Dan Katz is a grad of Bucknell Univ. in Pennsylvania.
Insight M (Sunnyvale, CA) – Aero detection of methane.
Odyssey (San Francisco, CA) – Mobile generative AI imaging systems. Tools for professional filmakers and animators.
Umbra Space (Santa Barbara, CA) – Remote sensing and high resultion imaging of Earth using satellites. cofounder David Langan has a BS from UC Irvine.
Ouster (San Jose, CA) – LIDAR systems. Founded by Angus Pacala and Mark Frichtl , both of whom have BS and MS degrees from Stanford
Network Optix (Walnut Creek, CA) – Video soultions with full-stack video platform and ecosystem.
Sensoride (San Diego, CA) – High resolution radar for vehicles.
Zendar (Berkeley, CA) – Radar for vehicles. Zendar was founded in 2017 by Vinayak Nagpal and Jimmy Wang, both Ph.Ds from UC Berkeley.
Observable Space (Los Angeles, CA) – Vertically integrated software and hardware telescopes and space imaging company. Founder Dan Roelker has a BS in math from Columbia Union College and Richard Hedrick has a BS in physics from UCLA.